Exam 9: Acids and Bases
Exam 1: Matter and Measurement89 Questions
Exam 2: Atoms and the Periodic Table90 Questions
Exam 3: Ionic Compounds90 Questions
Exam 4: Covalent Compounds90 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: Energy Changes, Reaction Rates, and Equilibrium88 Questions
Exam 7: Gases, Liquids, and Solids79 Questions
Exam 8: Solutions90 Questions
Exam 9: Acids and Bases90 Questions
Exam 10: Nuclear Chemistry85 Questions
Exam 11: Introduction to Organic Molecules and Functional Groups103 Questions
Exam 12: Alkanes106 Questions
Exam 13: Unsaturated Hydrocarbons101 Questions
Exam 14: Organic Compounds That Contain Oxygen, Halogen, or Sulfur111 Questions
Exam 15: The Three-Dimensional Shape of Molecules100 Questions
Exam 16: Aldehydes and Ketones103 Questions
Exam 17: Carboxylic Acids, Esters, and Amides81 Questions
Exam 18: Amines and Neurotransmitters105 Questions
Exam 19: Lipids105 Questions
Exam 20: Carbohydrates92 Questions
Exam 21: Amino Acids, Proteins, and Enzymes88 Questions
Exam 22: Nucleic Acids and Protein Synthesis89 Questions
Exam 23: Digestion and the Conversion of Food Into Energy92 Questions
Exam 24: Carbohydrate, Lipid, and Protein Metabolism91 Questions
Select questions type
The [-OH] in a sample of egg whites is 6.3 × 10-7 M. What is the [H3O+] in these egg whites?
(Multiple Choice)
4.8/5  (39)
(39)
What is the molarity of an HCl solution if 43.6 mL of a 0.125 M KOH solution are needed to titrate a 25.0 mL sample of the acid according to the equation below? KOH(aq)+ HCl(aq) H2O(l)+ KCl(aq)
(Multiple Choice)
4.9/5  (32)
(32)
In an acid-base reaction, a proton is transferred from the acid (HA)to the base (B:).
(True/False)
4.7/5  (42)
(42)
All salts where the anion is derived from a strong acid form basic solutions.
(True/False)
4.9/5  (48)
(48)
A strong acid readily donates a proton, forming a (strong/weak)_____ conjugate base.
(Short Answer)
4.8/5  (36)
(36)
A sample of urine has a pH of 8.2. Which statement describing the urine sample is NOT true?
(Multiple Choice)
4.8/5  (30)
(30)
What is the molarity of an HNO3 solution if 24.1 mL of a 0.250 M Ba(OH)2 solution are needed to titrate a 15.0 mL sample of the acid according to the equation below? Ba(OH)2(aq)+ 2 HNO3(aq) 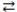 2 H2O(l)+ Ba(NO3)2(aq)
2 H2O(l)+ Ba(NO3)2(aq)
(Multiple Choice)
4.9/5  (52)
(52)
Ions that appear on both sides of an equation but undergo no change in a reaction are called _____ ions.
(Short Answer)
4.9/5  (31)
(31)
What is the net ionic equation for the acid-base reaction of hydrobromic acid with sodium hydroxide?
(Multiple Choice)
4.8/5  (40)
(40)
Two species that differ by the presence of a proton are called a conjugate acid-base pair.
(True/False)
4.7/5  (43)
(43)
An aqueous solution of KCl has a lower pH than an aqueous solution of Li2SO3.
(True/False)
4.9/5  (29)
(29)
A salt derived from a strong base and a weak acid forms an acidic solution.
(True/False)
4.9/5  (38)
(38)
Although a Brønsted-Lowry acid must contain a hydrogen atom, it may be a neutral molecule or contain a net positive or negative charge.
(True/False)
4.9/5  (42)
(42)
In the acid-base reaction: HCN(aq)+ F-(aq) 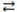 CN-(aq)+ HF(aq), where Ka (HCN)= 4.9 × 10-10 and Ka (HF)= 7.2 × 10-4, the
CN-(aq)+ HF(aq), where Ka (HCN)= 4.9 × 10-10 and Ka (HF)= 7.2 × 10-4, the
(Multiple Choice)
4.8/5  (39)
(39)
The pH of normal blood is 7.4. The pH of a diabetic's blood was determined to be 6.4. Which comparison of these two blood samples is accurate?
(Multiple Choice)
4.8/5  (43)
(43)
Showing 21 - 40 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)