Exam 9: Acids and Bases
Exam 1: Matter and Measurement89 Questions
Exam 2: Atoms and the Periodic Table90 Questions
Exam 3: Ionic Compounds90 Questions
Exam 4: Covalent Compounds90 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: Energy Changes, Reaction Rates, and Equilibrium88 Questions
Exam 7: Gases, Liquids, and Solids79 Questions
Exam 8: Solutions90 Questions
Exam 9: Acids and Bases90 Questions
Exam 10: Nuclear Chemistry85 Questions
Exam 11: Introduction to Organic Molecules and Functional Groups103 Questions
Exam 12: Alkanes106 Questions
Exam 13: Unsaturated Hydrocarbons101 Questions
Exam 14: Organic Compounds That Contain Oxygen, Halogen, or Sulfur111 Questions
Exam 15: The Three-Dimensional Shape of Molecules100 Questions
Exam 16: Aldehydes and Ketones103 Questions
Exam 17: Carboxylic Acids, Esters, and Amides81 Questions
Exam 18: Amines and Neurotransmitters105 Questions
Exam 19: Lipids105 Questions
Exam 20: Carbohydrates92 Questions
Exam 21: Amino Acids, Proteins, and Enzymes88 Questions
Exam 22: Nucleic Acids and Protein Synthesis89 Questions
Exam 23: Digestion and the Conversion of Food Into Energy92 Questions
Exam 24: Carbohydrate, Lipid, and Protein Metabolism91 Questions
Select questions type
The acid-base indicator phenolphthalein is colorless in acidic solutions and bright pink in basic solutions.
(True/False)
4.7/5  (37)
(37)
Consider two acids, A and B. If acid A is weaker than acid B, then acid B dissociates in water to a greater extent than acid A.
(True/False)
4.8/5  (36)
(36)
A sample of water from the Chesapeake Bay has [H3O+]=3.1 × 10-9 M. Which statement below accurately describes this water sample?
(Multiple Choice)
4.9/5  (42)
(42)
The reaction of a Brønsted-Lowry acid (HA)with the metal salt of a hydroxide base (MOH)is an example of a neutralization reaction-an acid-base reaction that produces a _____ and _____ as products.
(Short Answer)
4.8/5  (35)
(35)
The addition of a base to water decreases the pH of the solution.
(True/False)
4.9/5  (43)
(43)
The stronger the acid, the (larger/smaller)_____ the value of Ka.
(Short Answer)
4.9/5  (43)
(43)
When phosphoric acid (H3PO4)dissolves in water, the equilibrium shown below is established. If Ka = 7.5 × 10-3 for H3PO4, which statement concerning an aqueous solution of phosphoric acid is correct?
H3PO4 + H2O 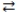 H3O+ + H2PO4-
H3O+ + H2PO4-
(Multiple Choice)
4.9/5  (43)
(43)
What is the pH of a buffer that contains 0.15 M CH3COOH and 0.10 M NaCH3COO (Ka = 1.8 × 10-5)?
(Multiple Choice)
4.9/5  (31)
(31)
The value of Kw applies to any aqueous solution at 25 °C , not just pure water.
(True/False)
4.8/5  (42)
(42)
The [H3O+] in a cabernet sauvignon wine is 5.9 × 10-4 M. What is the [-OH] in this wine?
(Multiple Choice)
4.9/5  (35)
(35)
Which salt forms a solution with a pH < 7 when dissolved in water?
(Multiple Choice)
4.9/5  (30)
(30)
Showing 61 - 80 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)