Exam 16: Liquids and Solids
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
The unit cell in a certain lattice consists of a cube formed by an anion at each corner, an anion in the center, and a cation at the center of each face. The unit cell contains a net
(Multiple Choice)
4.9/5  (40)
(40)
Mn crystallizes in the same cubic unit cell as Cu. Assuming that the radius of Mn is 5.6% larger than the radius of Cu and that the density of copper is 8.96 g/cm3, calculate the density of Mn.
(Multiple Choice)
4.9/5  (37)
(37)
A sample consisting of CO2(g) and CO2(s) at equilibrium at -78°C and 1 atm pressure is heated to -30°C, and the pressure is increased to 8 atm. Based on the phase diagram below, what will happen? 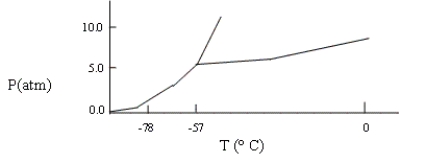
(Multiple Choice)
4.9/5  (41)
(41)
Shown below is a phase diagram for sulfur (not drawn to scale). Sulfur can exist in solid modifications, rhombic and monoclinic, denoted by SR and SM, respectively. Which of the following statements is incorrect? 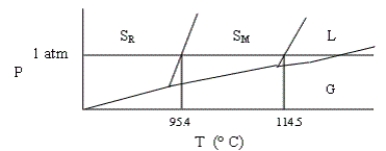
(Multiple Choice)
4.9/5  (42)
(42)
A metal crystallizes in a body-centered unit cell with an edge length of 2.18 102 pm. Assume the atoms in the cell touch along the cube diagonal. What will be the percentage of empty volume in the unit cell?
(Multiple Choice)
4.9/5  (40)
(40)
If equal, rigid spheres are arranged in a simple cubic lattice in the usual way (that is, in such a way that they touch each other), what fraction of the corresponding solid will be empty space? [The volume of a sphere is (4/3)r3, with = 3.14.]
(Multiple Choice)
4.9/5  (37)
(37)
A certain substance, X, has a triple-point temperature of 20°C at a pressure of 2.0 atm. Which one of the following statements cannot possibly be true?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following compounds has the lowest boiling point?
(Multiple Choice)
4.9/5  (45)
(45)
Which of the following chemical species has the highest boiling point?
(Multiple Choice)
4.8/5  (39)
(39)
Alkali halides commonly have either the sodium chloride structure or the cesium chloride structure. The molar mass of CsCl is 2.88 times the molar mass of NaCl, and the edge length of the unit cell for NaCl is 1.37 times the edge length of the CsCl unit cell. Determine the ratio of the density of CsCl to the density of NaCl.
(Multiple Choice)
4.9/5  (37)
(37)
Consider an ionic compound CxAy where the anions (A) are in a body-centered cubic arrangement and the cations (C) are located in all of the faces of the cubic unit cell.
A) What is the empirical formula of the salt?
B) If the edge length of the unit cell is 5.00 *10 cm and the molar masses of C and A are 50.0 g/mol and 100.0 g/mol, respectively, calculate the density of CxAy.
C) If the ionic radius of A is 200. pm, estimate the ionic radius of C.
(Short Answer)
4.9/5  (30)
(30)
Shown below is a phase diagram for compound X. At 25°C and 1 atm, in what state will X exist? 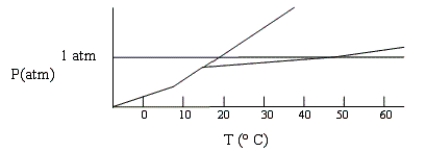
(Multiple Choice)
4.8/5  (31)
(31)
Which one of the following is the strongest intermolecular force experienced by noble gases?
(Multiple Choice)
4.8/5  (33)
(33)
What is the net number of face-centered atoms contained in a face-centered cubic unit cell?
(Multiple Choice)
4.7/5  (39)
(39)
The table below lists the ionic radii for the cations and anions in three ionic compounds.  Each compound has a cubic structure like NaCl, CsCl, or ZnS. Using radius ratios, predict the cubic structure that each compound is most likely to form (that of NaCl, CsCl, or ZnS), the type of holes filled by the cations, and the fraction of holes filled by cations.
Each compound has a cubic structure like NaCl, CsCl, or ZnS. Using radius ratios, predict the cubic structure that each compound is most likely to form (that of NaCl, CsCl, or ZnS), the type of holes filled by the cations, and the fraction of holes filled by cations.
(Essay)
4.9/5  (38)
(38)
Showing 41 - 60 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)