Exam 16: Liquids and Solids
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. A large increase in pressure will
(Multiple Choice)
4.7/5  (39)
(39)
The heat of combustion of bituminous coal is 2.5 *104 J/g. What quantity of the coal is required to produce the energy to convert 100. lb of ice at 0°C to steam at 100°C?
(Short Answer)
4.8/5  (37)
(37)
The elements of Group 5A, the nitrogen family, form compounds with hydrogen that have the boiling points listed below. SbH3 -17°C, AsH3 -55°C, PH3 -87°C, NH3 -33°C
The first three elements illustrate a trend where the boiling point decreases as the mass decreases; however, ammonia (NH3) does not follow the trend because of
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following statements is true about p-type silicon?
(Multiple Choice)
4.7/5  (37)
(37)
In which of the following processes is energy evolved as heat?
(Multiple Choice)
4.8/5  (37)
(37)
Metallic copper crystallizes in a face-centered cubic lattice. The volume of the unit cell is 4.11 108 pm3. What is the density of copper metal?
(Multiple Choice)
4.7/5  (36)
(36)
You are given the following boiling-point data: water, H2O
100° C
Methanol, CH3OH
64)96° C
Ethanol, CH3CH2OH
78)5° C
Diethyl ether, CH3OH2-O-CH2CH3
34)5° C
Ethylene glycol, HO-CH2-CH2-OH
198° C
Which one of these liquids would you expect to have the highest vapor pressure at room temperature?
(Multiple Choice)
4.7/5  (41)
(41)
Given the graph below, what is the boiling point of carbon tetrachloride at standard pressure? 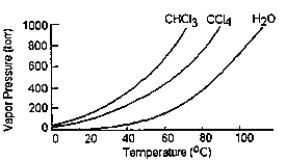
(Multiple Choice)
5.0/5  (48)
(48)
Which one of the following statements about solid Cu (face-centered cubic unit cell) is incorrect?
(Multiple Choice)
4.7/5  (41)
(41)
Shown below is a phase diagram for compound X. How will the melting point of X change with increased pressure? 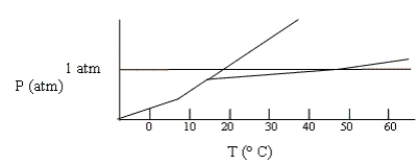
(Multiple Choice)
4.8/5  (47)
(47)
MnO has either a structure like NaCl or a structure like CsCl. The edge length of the MnO unit cell is 4.47 *10-8 cm, and the density of MnO is 5.28 g/cm3.
A) Does MnO crystallize like NaCl or like CsCl?
B) If the ionic radius of oxygen is 140. pm, estimate the ionic radius of manganese.
C) Does the calculated ratio of the cation radius to the anion radius for MnO support your answer in part A? Explain.
(Essay)
4.9/5  (35)
(35)
How many grams of ice would be melted by the energy obtained as 18.0 g of steam is condensed at 100°C and cooled to 0°C?
(Short Answer)
4.8/5  (36)
(36)
A certain compound with a molar mass of 138.0 g/mol crystallizes with the sodium chloride (rock salt) structure. The length of an edge of the unit cell is 488 pm. What is the density of this compound?
(Multiple Choice)
4.9/5  (39)
(39)
Below is a phase diagram for compound X. You wish to purify a sample of X that was collected at P = 1.0 atm and T = 100 by subliming it. In order to sublime the sample, you should 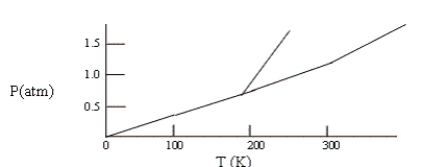
(Multiple Choice)
4.7/5  (31)
(31)
Below is a phase diagram for compound X. What is the normal boiling point of X is most likely to be? 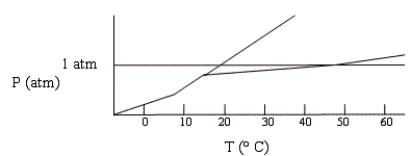
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following is the smallest hole in a closest-packed lattice of spheres?
(Multiple Choice)
4.8/5  (37)
(37)
Which is generally larger, the heat of fusion or the heat of vaporization for a given substance? Explain your answer.
(Essay)
4.9/5  (33)
(33)
Showing 81 - 100 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)