Exam 16: Liquids and Solids
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
Aluminum metal crystallizes in a face-centered cubic structure. What is the relationship between the radius of an Al atom (r) and the length of an edge of the unit cell (E)?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following should have the highest boiling point?
(Multiple Choice)
4.9/5  (39)
(39)
Hvap for water is 40.7 kJ/mol. Calculate the boiling point of water at 0.500 atm.
(Multiple Choice)
4.8/5  (40)
(40)
The molar volume of a certain form of solid lead is 18 cm3/mol. Assuming cubic closest-packed structure, determine the following.
-The number of Pb atoms per unit cell
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following substances would you expect to have the lowest boiling point?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the compounds below is an example of a network solid?
(Multiple Choice)
4.8/5  (39)
(39)
A metal crystallizes with a face-centered cubic lattice. The edge of the unit cell is 408 pm. What is the diameter of the metal atom?
(Multiple Choice)
4.9/5  (37)
(37)
Elemental magnesium crystallizes in a face-centered cubic lattice. The density of magnesium is 1.738 g/cm3. The unit cell length is 4.80 102 pm. What is the atomic radius of Mg?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following statements is true about the vapor pressures of methane (CH4) and ammonia (NH3)?
(Multiple Choice)
4.8/5  (36)
(36)
A sample of Co crystallizes in the hexagonal closest-packed (hcp) structure. Each atom in a layer is surrounded by and touches 6 other Co atoms. If the distance between Co atom centers within each layer is 2.0 102 pm, what is the distance between centers of nearest neighbors in adjacent layers?
(Multiple Choice)
4.9/5  (39)
(39)
Given the phase diagram shown below, which of the following statements is false? 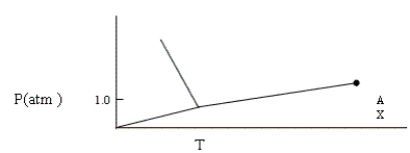
(Multiple Choice)
4.8/5  (38)
(38)
How much energy is needed to convert 64.6 grams of ice at 0.00°C to water at 55.2°C? Specific heat of ice = 2.10 J/(g°C)
Specific heat of water = 4.18 J/(g°C)
Heat of fusion = 333 J/g
Heat of vaporization = 2258 J/g
(Multiple Choice)
4.7/5  (32)
(32)
Doping Se with As would produce a(n) __________ semiconductor with __________ conductivity compared to pure Se.
(Multiple Choice)
4.7/5  (36)
(36)
Given below are the temperatures at which two different liquid compounds with the same empirical formula have a vapor pressure of 400 torr. 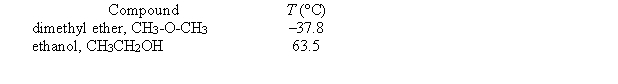 Which of the following statements is false?
Which of the following statements is false?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 21 - 40 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)