Exam 16: Liquids and Solids
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
Based on intermolecular forces, which of the following will have the highest boiling point?
(Multiple Choice)
4.8/5  (36)
(36)
On the basis of your knowledge of bonding in liquids and solids, which of the following substances has the highest melting temperature?
(Multiple Choice)
4.9/5  (34)
(34)
The table below lists the ionic radii for the cations and anions in three ionic compounds.  Each compound has a cubic structure like NaCl, CsCl, or ZnS. Use the ratio of cation radius to anion radius to predict the structure formed (that of NaCl, CsCl, or ZnS). Then estimate the density of each compound. For ZnS structures, the holes occupied lie along the body diagonals of the unit cell so that 1/4 (body diagonal) = r+ + r-.
Each compound has a cubic structure like NaCl, CsCl, or ZnS. Use the ratio of cation radius to anion radius to predict the structure formed (that of NaCl, CsCl, or ZnS). Then estimate the density of each compound. For ZnS structures, the holes occupied lie along the body diagonals of the unit cell so that 1/4 (body diagonal) = r+ + r-.
(Essay)
4.9/5  (34)
(34)
Make a sketch to show the hydrogen bonding between two acetic acid molecules (HC2H3O2).
(Essay)
4.9/5  (40)
(40)
The unit cell in this two-dimensional crystal contains __________ Xs and __________ Os. 
(Multiple Choice)
4.8/5  (25)
(25)
What is the radius of the largest sphere that can be placed at the center of a face-centered cubic unit cell of a cubic closest-packed array of spheres if the spheres have diameters of 4.00 102 pm?
(Multiple Choice)
4.8/5  (31)
(31)
Knowing that Hvap for water is 40.7 kJ/mol, calculate Pvap of water at 37°C.
(Multiple Choice)
4.7/5  (38)
(38)
Which of the following chemical species has the highest boiling point?
(Multiple Choice)
4.8/5  (35)
(35)
100. g of ice at 0°C is added to 300.0 g of water at 60°C. Assuming no transfer of heat to the surroundings, what is the temperature of the liquid water after all the ice has melted?
(Short Answer)
4.8/5  (44)
(44)
In the unit cell of sphalerite, Zn2+ ions occupy half the tetrahedral holes in a face-centered cubic lattice of S2- ions. What is the number of formula units of ZnS in the unit cell?
(Multiple Choice)
4.8/5  (34)
(34)
KCl crystallizes in a structure like NaCl. The ionic radius of Na+ is 0.525 times the ionic radius of Cl- and 0.714 times the ionic radius of K+.
A) Estimate the ratio of the unit cell edge length for KCl to that for NaCl.
B) Estimate the ratio of the density of NaCl to that of KCl.
(Short Answer)
4.8/5  (33)
(33)
Based on the phase diagram shown below, which of the following statements are correct?
I. Sublimation occurs at a point in the transformation that falls along a straight line from point A to point F.
II. C and E represent points where the gas and liquid phases are in equilibrium.
III. (Hvap can be measured at point B).
IV. Molecules at point D have a greater average kinetic energy than those at point F.
V. The temperature at point E is called the critical temperature of the compound. 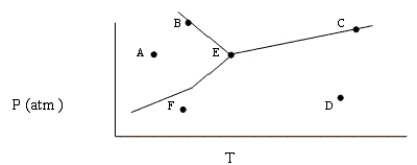
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following statements is(are) false?
I.The hexagonal closest-packed structure is ABAB....
II.A body-centered cubic unit cell has four atoms per unit cell.
III.For unit cells having the same edge length, a simple cubic structure would have a smaller density than a body-centered cube.
IV.Atoms in a solid consisting of only one element would have six nearest neighbors if the crystal structure was a simple cubic array.
(Multiple Choice)
4.9/5  (32)
(32)
The triple point of CO2 is at 5.2 atm and -57°C. Under atmospheric conditions present in a typical Boulder, Colorado, laboratory (P = 630 torr, T = 23°C), solid CO2 will
(Multiple Choice)
4.9/5  (38)
(38)
The molar volume of a certain form of solid lead is 18 cm3/mol. Assuming cubic closest-packed structure, determine the following.
-The volume of a single cell
(Multiple Choice)
4.8/5  (45)
(45)
Pure rubidium crystallizes in a body-centered cubic lattice; the edge length of the unit cell is 562 pm. What is the density of rubidium in grams per cubic centimeter?
(Multiple Choice)
4.8/5  (33)
(33)
A material is made from Al, Ga, and As. The mole fractions of these elements are 0.25, 0.26, and 0.49, respectively. This material would be
(Multiple Choice)
4.9/5  (42)
(42)
The normal boiling point of liquid X is less than that of Y, which is less than that of Z. Which of the following is the correct order of increasing vapor pressure of the three liquids at STP?
(Multiple Choice)
4.9/5  (39)
(39)
The heat of vaporization of a certain refrigerant is 148 J/g. Calculate the number of kilograms of refrigerant that must be evaporated to freeze a tray of 16 one-ounce (1 oz = 28 g) ice cubes starting with the water at 15°C. Heat capacity (water) = 4.184 J/g °C
Hfusion (water) = 333 J/g
(Multiple Choice)
4.7/5  (38)
(38)
Showing 61 - 80 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)