Exam 18: Temperature, Heat, and the First Law of Thermodynamics
Exam 1: Measurement37 Questions
Exam 2: Motion Along a Straight Line90 Questions
Exam 3: Vector37 Questions
Exam 4: Motion in Two and Three Dimensions56 Questions
Exam 5: Force and Motion I73 Questions
Exam 6: Force and Motion II74 Questions
Exam 7: Kinetic Energy and Work73 Questions
Exam 8: Potential Energy and Conservation of Energy63 Questions
Exam 9: Center of Mass and Linear Momentum99 Questions
Exam 10: Rotation102 Questions
Exam 11: Rolling, Torque, and Angular Momentum66 Questions
Exam 12: Equilibrium and Elasticity57 Questions
Exam 13: Gravitation55 Questions
Exam 14: Fluids88 Questions
Exam 15: Oscillations75 Questions
Exam 16: Waves I82 Questions
Exam 17: Waves II71 Questions
Exam 18: Temperature, Heat, and the First Law of Thermodynamics96 Questions
Exam 19: The Kinetic Theory of Gases113 Questions
Exam 20: Entropy and the Second Law of Thermodynamics61 Questions
Exam 21: Electric Charge52 Questions
Exam 22: Electric Fields55 Questions
Exam 23: Gauss Law38 Questions
Exam 24: Electric Potential52 Questions
Exam 25: Capacitance61 Questions
Exam 26: Current and Resistance55 Questions
Exam 27: Circuits73 Questions
Exam 28: Magnetic Fields55 Questions
Exam 29: Magnetic Fields Due to Currents49 Questions
Exam 30: Induction and Inductance90 Questions
Exam 31: Electromagnetic Oscillations and Alternating Current88 Questions
Exam 32: Maxwells Equations; Magnetism of Matter81 Questions
Exam 33: Electromagnetic Waves83 Questions
Exam 34: Images79 Questions
Exam 35: Interference46 Questions
Exam 36: Diffraction77 Questions
Exam 37: Relativity68 Questions
Exam 38: Photons and Matter Waves57 Questions
Exam 39: More About Matter Waves41 Questions
Exam 40: All About Atoms79 Questions
Exam 41: Conduction of Electricity in Solids51 Questions
Exam 42: Nuclear Physics68 Questions
Exam 43: Energy From the Nucleus50 Questions
Exam 44: Quarks, Leptons, and the Big Bang55 Questions
Select questions type
When a certain constant volume gas thermometer is in thermal contact with water at its triple point (273.16 K) the pressure is 6.30 * 104 Pa. For this thermometer a kelvin corresponds to a change in pressure of about:
(Multiple Choice)
4.9/5  (37)
(37)
In the first law of thermodynamics, the sign of the heat transfer Q:
(Multiple Choice)
4.9/5  (34)
(34)
During the time that latent heat is involved in a change of state:
(Multiple Choice)
4.9/5  (31)
(31)
The energy given off by 300 grams of an alloy as it cools by 50 C raises the temperature of 300 grams of water from 30 C to 40 C. The specific heat of the alloy is:
(Multiple Choice)
4.7/5  (37)
(37)
Solid A, with mass M, is at its melting point TA. It is placed in thermal contact with solid B, with heat capacity CB and initially at temperature TB (TB > TA). The combination is thermally isolated. A has latent heat of fusion L and when it has melted has heat capacity CA. If A completely melts the final temperature of both A and B is:
(Multiple Choice)
4.8/5  (38)
(38)
In the figure, a gas undergoes a transition from point A to point B along the path shown. How much work is done by the gas? 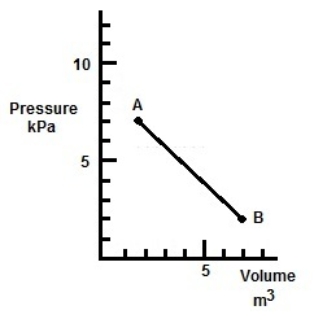
(Multiple Choice)
4.9/5  (40)
(40)
Possible units for the coefficient of volume expansion are:
(Multiple Choice)
4.9/5  (30)
(30)
A metal sample of mass M requires a power input P to just remain molten. When the heater is turned off, the metal solidifies in a time T. The specific latent heat of fusion of this metal is:
(Multiple Choice)
4.9/5  (26)
(26)
Fahrenheit and Kelvin scales agree numerically at a reading of:
(Multiple Choice)
5.0/5  (44)
(44)
Metal pipes, used to carry water, sometimes burst in the winter because:
(Multiple Choice)
4.7/5  (33)
(33)
A cube of aluminum has an edge length of 20 cm. Aluminum has a density 2.7 times that of water (1 g/cm3) and a specific heat 0.217 times that of water (1 cal/g.C˚). When the internal energy of the cube increases by 47000 cal its temperature increases by:
(Multiple Choice)
4.8/5  (43)
(43)
The rate of heat flow by conduction through a slab does NOT depend upon the:
(Multiple Choice)
4.9/5  (37)
(37)
Showing 81 - 96 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)