Exam 18: Temperature, Heat, and the First Law of Thermodynamics
Exam 1: Measurement37 Questions
Exam 2: Motion Along a Straight Line90 Questions
Exam 3: Vector37 Questions
Exam 4: Motion in Two and Three Dimensions56 Questions
Exam 5: Force and Motion I73 Questions
Exam 6: Force and Motion II74 Questions
Exam 7: Kinetic Energy and Work73 Questions
Exam 8: Potential Energy and Conservation of Energy63 Questions
Exam 9: Center of Mass and Linear Momentum99 Questions
Exam 10: Rotation102 Questions
Exam 11: Rolling, Torque, and Angular Momentum66 Questions
Exam 12: Equilibrium and Elasticity57 Questions
Exam 13: Gravitation55 Questions
Exam 14: Fluids88 Questions
Exam 15: Oscillations75 Questions
Exam 16: Waves I82 Questions
Exam 17: Waves II71 Questions
Exam 18: Temperature, Heat, and the First Law of Thermodynamics96 Questions
Exam 19: The Kinetic Theory of Gases113 Questions
Exam 20: Entropy and the Second Law of Thermodynamics61 Questions
Exam 21: Electric Charge52 Questions
Exam 22: Electric Fields55 Questions
Exam 23: Gauss Law38 Questions
Exam 24: Electric Potential52 Questions
Exam 25: Capacitance61 Questions
Exam 26: Current and Resistance55 Questions
Exam 27: Circuits73 Questions
Exam 28: Magnetic Fields55 Questions
Exam 29: Magnetic Fields Due to Currents49 Questions
Exam 30: Induction and Inductance90 Questions
Exam 31: Electromagnetic Oscillations and Alternating Current88 Questions
Exam 32: Maxwells Equations; Magnetism of Matter81 Questions
Exam 33: Electromagnetic Waves83 Questions
Exam 34: Images79 Questions
Exam 35: Interference46 Questions
Exam 36: Diffraction77 Questions
Exam 37: Relativity68 Questions
Exam 38: Photons and Matter Waves57 Questions
Exam 39: More About Matter Waves41 Questions
Exam 40: All About Atoms79 Questions
Exam 41: Conduction of Electricity in Solids51 Questions
Exam 42: Nuclear Physics68 Questions
Exam 43: Energy From the Nucleus50 Questions
Exam 44: Quarks, Leptons, and the Big Bang55 Questions
Select questions type
A Kelvin thermometer and a Fahrenheit thermometer both give the same reading for a certain sample. The corresponding Celsius temperature is:
(Multiple Choice)
4.8/5  (35)
(35)
An electric stove burner of diameter 20 cm is at a temperature of 250 °C. If σ = 5.67 x 10-8 W/m2·K4, at what rate is the burner radiating energy? Assume the emissivity ε = 0.6.
(Multiple Choice)
4.7/5  (37)
(37)
A balloon is filled with cold air and placed in a warm room. It is NOT in thermal equilibrium with the air of the room until
(Multiple Choice)
4.8/5  (35)
(35)
The coefficient of linear expansion of steel is 11 *10-6 per C . A steel ball has a volume of exactly 100 cm3 at 0 C. When heated to 100 C, its volume becomes:
(Multiple Choice)
4.9/5  (35)
(35)
Thin strips of iron and zinc are riveted together to form a bimetallic strip which bends when heated. The iron is on the inside of the bend because:
(Multiple Choice)
4.7/5  (37)
(37)
The heat of fusion of water is 79.5 cal/g. This means 79.5 cal of energy are required to:
(Multiple Choice)
4.9/5  (31)
(31)
A system undergoes an adiabatic process in which its internal energy increases by 20 J. Which of the following statements is true?
(Multiple Choice)
4.8/5  (37)
(37)
How many calories are required to change one gram of 0 C ice to 100 C steam? The latent heat of fusion is 80 cal/g and the latent heat of vaporization is 540 cal/g. The specific heat of water is 1.00 cal/g . K.
(Multiple Choice)
4.9/5  (35)
(35)
The specific heat of lead is 0.030 cal/g . C. 300 g of lead shot at 100 C is mixed with 100 g of water at 70 C in an insulated container. The final temperature of the mixture is:
(Multiple Choice)
4.8/5  (34)
(34)
The Stanford linear accelerator contains hundreds of brass disks tightly fitted into a steel tube (see figure). The coefficient of linear expansion of the brass is 2.00 *10-5 per C . The system was assembled by cooling the disks in dry ice (-57 C) to enable them to just slide into the close-fitting tube. If the diameter of a disk is 80.00 mm at 43 C, what is its diameter in the dry ice? 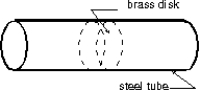
(Multiple Choice)
4.9/5  (39)
(39)
Pressure vs. volume graphs for a certain gas undergoing five different cyclic processes are shown below. During which cycle does the gas do the greatest positive work? 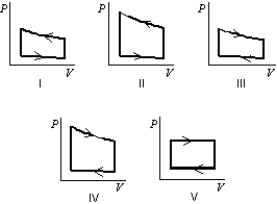
(Multiple Choice)
4.9/5  (31)
(31)
One degree is the same on the following temperature scales:
(Multiple Choice)
4.8/5  (28)
(28)
An iron stove, used for heating a room by radiation, is more efficient if:
(Multiple Choice)
4.7/5  (36)
(36)
Showing 61 - 80 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)