Exam 18: Temperature, Heat, and the First Law of Thermodynamics
Exam 1: Measurement37 Questions
Exam 2: Motion Along a Straight Line90 Questions
Exam 3: Vector37 Questions
Exam 4: Motion in Two and Three Dimensions56 Questions
Exam 5: Force and Motion I73 Questions
Exam 6: Force and Motion II74 Questions
Exam 7: Kinetic Energy and Work73 Questions
Exam 8: Potential Energy and Conservation of Energy63 Questions
Exam 9: Center of Mass and Linear Momentum99 Questions
Exam 10: Rotation102 Questions
Exam 11: Rolling, Torque, and Angular Momentum66 Questions
Exam 12: Equilibrium and Elasticity57 Questions
Exam 13: Gravitation55 Questions
Exam 14: Fluids88 Questions
Exam 15: Oscillations75 Questions
Exam 16: Waves I82 Questions
Exam 17: Waves II71 Questions
Exam 18: Temperature, Heat, and the First Law of Thermodynamics96 Questions
Exam 19: The Kinetic Theory of Gases113 Questions
Exam 20: Entropy and the Second Law of Thermodynamics61 Questions
Exam 21: Electric Charge52 Questions
Exam 22: Electric Fields55 Questions
Exam 23: Gauss Law38 Questions
Exam 24: Electric Potential52 Questions
Exam 25: Capacitance61 Questions
Exam 26: Current and Resistance55 Questions
Exam 27: Circuits73 Questions
Exam 28: Magnetic Fields55 Questions
Exam 29: Magnetic Fields Due to Currents49 Questions
Exam 30: Induction and Inductance90 Questions
Exam 31: Electromagnetic Oscillations and Alternating Current88 Questions
Exam 32: Maxwells Equations; Magnetism of Matter81 Questions
Exam 33: Electromagnetic Waves83 Questions
Exam 34: Images79 Questions
Exam 35: Interference46 Questions
Exam 36: Diffraction77 Questions
Exam 37: Relativity68 Questions
Exam 38: Photons and Matter Waves57 Questions
Exam 39: More About Matter Waves41 Questions
Exam 40: All About Atoms79 Questions
Exam 41: Conduction of Electricity in Solids51 Questions
Exam 42: Nuclear Physics68 Questions
Exam 43: Energy From the Nucleus50 Questions
Exam 44: Quarks, Leptons, and the Big Bang55 Questions
Select questions type
In the figure, what is the sign of the work done by the gas? 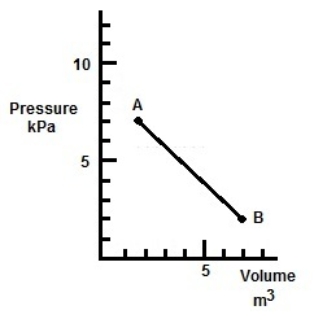
(Multiple Choice)
4.9/5  (33)
(33)
If the zeroth law of thermodynamics were not valid, which of the following could not be considered a property of an object?
(Multiple Choice)
4.9/5  (42)
(42)
It is more difficult to measure the coefficient of volume expansion of a liquid than that of a solid because:
(Multiple Choice)
4.8/5  (37)
(37)
The diagram shows four rectangular plates and their dimensions. All are made of the same material. The temperature now increases. Of these plates: 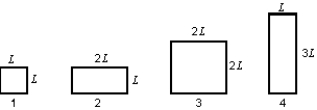
(Multiple Choice)
4.8/5  (36)
(36)
The diagram shows four slabs of different materials with equal thickness, placed side by side. Heat flows from left to right and the steady-state temperatures of the interfaces are given. Rank the materials according to their rates of thermal conduction, smallest to largest. 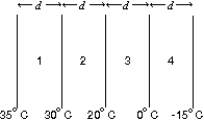
(Multiple Choice)
4.8/5  (40)
(40)
The coefficient of linear expansion of iron is 10-5 per C . The volume of an iron cube, 5 cm on edge, will increase by what amount if it is heated from 10 C to 60 C?
(Multiple Choice)
4.8/5  (35)
(35)
Fifty grams of ice at 0 C is placed in a thermos bottle containing one hundred grams of water at 6 C. How many grams of ice will melt? The heat of fusion of water is 333 kJ/kg and the specific heat of water is 4190 J/kg . K.
(Multiple Choice)
4.7/5  (26)
(26)
There is a temperature at which the reading on the Kelvin scale is numerically:
(Multiple Choice)
4.9/5  (30)
(30)
During an adiabatic process an object does 100 J of work and its temperature decreases by 5 K. During another process it does 25 J of work and its temperature decreases by 5 K. Its heat capacity for the second process is:
(Multiple Choice)
4.8/5  (33)
(33)
The mercury column in an ordinary medical thermometer doubles in length when its temperature changes from 95 F to 105 F. Choose the correct statement:
(Multiple Choice)
4.8/5  (41)
(41)
Steam at 100 C enters a radiator and leaves as water (at 80 C). Take the heat of vaporization to be 540 cal/g. Of the total energy given off as heat, what percent arises from the cooling of the water?
(Multiple Choice)
4.9/5  (40)
(40)
The "triple point" of a substance is that point for which the temperature and pressure are such that:
(Multiple Choice)
4.9/5  (28)
(28)
A slab of material has area A, thickness L, and thermal conductivity k. One of its surfaces (P) is maintained at temperature T1 and the other surface (Q) is maintained at a lower temperature T2. The rate of heat flow from P to Q is:
(Multiple Choice)
4.9/5  (40)
(40)
When two gases separated by a diathermal wall are in thermal equilibrium with each other:
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following statements pertaining to a vacuum flask (thermos) is NOT correct?
(Multiple Choice)
4.8/5  (31)
(31)
The diagram shows four slabs of different materials with equal thickness, placed side by side. Heat flows from left to right and the steady-state temperatures of the interfaces are given. Rank the materials according to their thermal conductivities, smallest to largest. 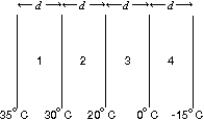
(Multiple Choice)
4.9/5  (31)
(31)
Showing 21 - 40 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)