Exam 5: Methods in Protein Biochemistry
Exam 1: Principles of Biochemistry100 Questions
Exam 2: Physical Biochemistry: Energy Conversion, Water, and Membranes100 Questions
Exam 3: Nucleic Acid Structure and Function100 Questions
Exam 4: Protein Structure100 Questions
Exam 5: Methods in Protein Biochemistry100 Questions
Exam 6: Protein Function114 Questions
Exam 7: Enzyme Mechanisms106 Questions
Exam 8: Cell Signaling Systems102 Questions
Exam 9: Glycolysis: a Paradigm of Metabolic Regulation100 Questions
Exam 10: The Citrate Cycle100 Questions
Exam 11: Oxidative Phosphorylation99 Questions
Exam 12: Photosynthesis100 Questions
Exam 13: Carbohydrate Structure and Function100 Questions
Exam 14: Carbohydrate Metabolism100 Questions
Exam 15: Lipid Structure and Function100 Questions
Exam 16: Lipid Metabolism100 Questions
Exam 17: Amino Acid Metabolism100 Questions
Exam 18: Nucleotide Metabolism99 Questions
Exam 19: Metabolic Integration101 Questions
Exam 20: DNA Replication, Repair, and Recombination99 Questions
Exam 21: RNA Synthesis, Processing, and Gene Silencing100 Questions
Exam 22: Protein Synthesis, Posttranslational Modification, and Transport100 Questions
Exam 23: Gene Regulation100 Questions
Select questions type
Which component used in a Western blot is covalently linked to an enzyme?
(Multiple Choice)
4.8/5  (30)
(30)
Calculate the specific activity when 500 mg of protein has an activity of 18,000 units.
(Multiple Choice)
4.7/5  (36)
(36)
Which technique ionizes polypeptides by embedding the tryptic fragments into a light-absorbing matrix and exposing it to a laser?
(Multiple Choice)
4.9/5  (40)
(40)
Compare and contrast electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI).
(Essay)
4.9/5  (45)
(45)
After using the salting out method to isolate proteins, how is ammonium sulfate removed from the solution?
(Multiple Choice)
4.8/5  (25)
(25)
Using the figure below describing monoclonal antibody generation, identify the step where the B cells are fused with the tumor cells. 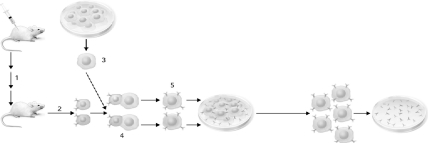
(Multiple Choice)
4.8/5  (31)
(31)
The part of the Western blot that contains the protein-specific recognition and facilitates the antigen-antibody interactions is the
(Multiple Choice)
4.8/5  (36)
(36)
During solid-phase peptide synthesis, 9-fluorenylmethoxycarbonyl is used
(Multiple Choice)
4.8/5  (33)
(33)
The figure below shows three proteins that are separated using gel filtration chromatography. Which protein is the largest? 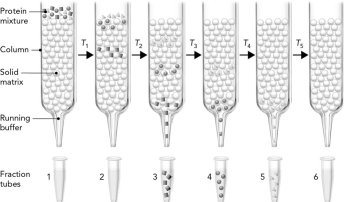
(Multiple Choice)
4.7/5  (39)
(39)
Describe the major difference between the types of protein samples used in X-ray crystallography and NMR spectroscopy. Then explain how this difference limits each method.
(Essay)
4.9/5  (38)
(38)
Below are the steps of solid-phase peptide synthesis: 1. The protecting groups of the amino acids are removed.
2) The peptide is released from the solid support resin.
3) An incoming amino acid is activated at the carboxyl group by DCC and added to the column.
4) Fmoc is removed by treatment with a base and the C-terminal amino acid is attached to the resin molecule.
"5) The resin bound C-terminal amino acid and the incoming N-terminal amino acid are coupled.
What is the appropriate order of these steps?"
(Multiple Choice)
4.9/5  (33)
(33)
Which enzyme or reagent cleaves a peptide at the carboxyl side of a methionine residue?
(Multiple Choice)
4.9/5  (32)
(32)
The step in solid-phase peptide synthesis that occurs at number 3 is 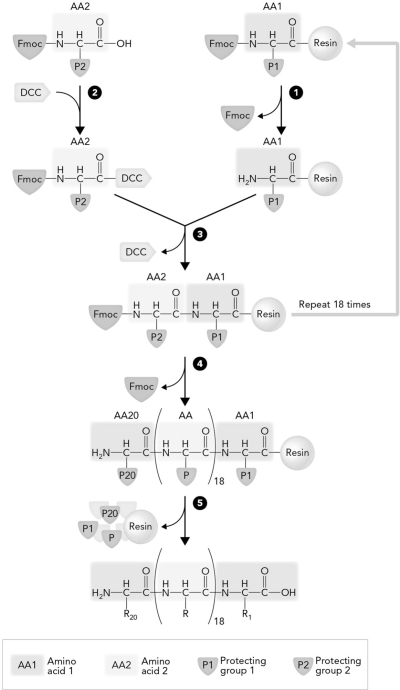
(Multiple Choice)
5.0/5  (42)
(42)
Compare and contrast the three MOST commonly used homogenization techniques to prepare a cell extract.
(Essay)
4.7/5  (37)
(37)
Protein NMR is more useful than X-ray crystallography for studying
(Multiple Choice)
4.8/5  (37)
(37)
Predict the fragments of the following peptide after cleavage by trypsin. GLMKTYPDSTA
(Multiple Choice)
4.9/5  (40)
(40)
Showing 41 - 60 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)