Exam 4: Protein Structure and Function
Exam 1: Cells: The Fundamental Units of Life64 Questions
Exam 2: Chemical Components of Cells74 Questions
Exam 3: Energy, Catalysis, and Biosynthesis73 Questions
Exam 4: Protein Structure and Function71 Questions
Exam 5: DNA and Chromosomes69 Questions
Exam 6: DNA Replication and Repair61 Questions
Exam 7: From DNA to Protein62 Questions
Exam 8: Control of Gene Expression68 Questions
Exam 9: How Genes and Genomes Evolve60 Questions
Exam 10: Analyzing the Structure and Function of Genes59 Questions
Exam 11: Membrane Structure57 Questions
Exam 12: Transport Across Cell Membranes67 Questions
Exam 13: How Cells Obtain Energy From Food71 Questions
Exam 14: Energy Generation in Mitochondria and Chloroplasts72 Questions
Exam 15: Intracellular Compartments and Protein Transport55 Questions
Exam 16: Cell Signaling60 Questions
Exam 17: Cytoskeleton59 Questions
Exam 18: The Cell-Division Cycle67 Questions
Exam 19: Sexual Reproduction and the Power of Genetics61 Questions
Exam 20: Cell Communities: Tissues, Stem Cells, and Cancer57 Questions
Select questions type
For each of the following sentences, fill in the blanks with the best word or phrase selected from the list below.Not all words or phrases will be used; each word or phrase should be used only once.
activation energy inhibitors products active site isomers substrates free energy ligand transition state high-energy low-energy
Any substance that will bind to a protein is known as its __________.Enzymes bind their __________ at the __________.The enzyme hexokinase is so specific that it reacts with only one of the two __________ of glucose.Enzymes catalyze a chemical reaction by lowering the __________, because they provide conditions favorable for the formation of a __________ intermediate called the __________.Once the reaction is completed, the enzyme releases the __________ of the reaction.
(Essay)
4.8/5  (33)
(33)
Which of the following mechanisms best describes the manner in which lysozyme lowers the energy required for its substrate to reach its transition-state conformation?
(Multiple Choice)
4.7/5  (36)
(36)
Figure 4-78 shows a fatty-acid-binding protein from two different angles.Apart from its two short α helices, its structural elements are extended strands that form a curved β sheet, which is called a β barrel.
A.Draw arrows on the six top strands in panel (A) (those running horizontally) to determine whether the β barrel is made up of parallel or antiparallel strands.
B.Look at panel (B) and predict the relative distribution of polar and nonpolar side chains (inside the barrel or outside the barrel) and explain your answer. 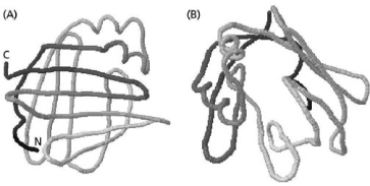 Figure 4-78
Figure 4-78
(Essay)
4.8/5  (41)
(41)
Using the example of the p53 protein, postulate how different combinations of covalent modifications can lead to a wide variety of protein functions.
(Essay)
4.9/5  (33)
(33)
Which of the following is NOT a feature commonly observed in α helices?
(Multiple Choice)
4.8/5  (46)
(46)
Protein Y is a globular protein that normally assembles as a tetramer.You are examining the interactions between the subunits by changing the amino acids on the surface of the protein.You compare the wild-type (nonmutated) protein and a mutant version with a single amino acid substitution.When washed through the same gel-filtration column, mutant protein Y runs through the column more slowly than the normal protein.Which of the following changes in the mutant protein is most likely to explain this result? Explain your choice.
A.the loss of a binding site on the mutant-protein surface through which protein Y normally forms dimers
B.a change that results in the mutant protein acquiring an overall positive instead of a negative charge
C.a change that results in the mutant protein being larger than the wild-type protein
D.a change that results in the mutant protein having a slightly different shape from the wild-type protein
(Essay)
4.8/5  (40)
(40)
For each of the following sentences, fill in the blanks with the best word or phrase selected from the list below.Not all words or phrases will be used; each word or phrase should be used only once.
affinity billions ligands antibodies coiled-coils loops antigens hundreds size-exclusion \beta strands ion-exchange
The human immune system produces __________ of different immunoglobulins, also called __________, which enable the immune system to recognize and fight germs by specifically binding one or a few related __________.The hypervariable structural element that forms the ligand-binding site is comprised of several __________.Purified antibodies are useful for a variety of experimental purposes, including protein purification using __________ chromatography.
(Essay)
4.9/5  (35)
(35)
One of the key features of living systems is the use of energy to create and maintain order.A good example is found in the folding of newly synthesized proteins.Which activated carrier molecule is used by chaperone proteins to support protein folding?
(Multiple Choice)
4.8/5  (45)
(45)
Enzymes generally make good drug targets because a specific reaction of interest can be targeted with a high degree of selectivity.Consider the following three drugs and explain why, although reaction-specific, the first two produce side effects, while the third does not.
A.Statins inhibit HMG CoA reductase to block intracellular cholesterol synthesis.
B.Methotrexate inhibits dihydrofolate reductase, which subsequently leads to blocked DNA replication.
C.Gleevec® inhibits BCR, a kinase that is only produced in certain types of leukemia cells.
(Essay)
4.9/5  (38)
(38)
The phosphorylation of a protein is typically associated with a change in activity, the assembly of a protein complex, or the triggering of a downstream signaling cascade.The addition of ubiquitin, a small polypeptide, is another type of covalent modification that can affect the protein function.Ubiquitylation often results in
(Multiple Choice)
4.8/5  (38)
(38)
Coiled-coils are typically found in proteins that require an elongated structural framework.Which of the following proteins do you expect to have a coiled-coil domain?
(Multiple Choice)
4.9/5  (37)
(37)
Some of the enzymes that oxidize sugars to yield usable cellular energy (for example, ATP) are regulated by phosphorylation.For these enzymes, would you expect the inactive form to be the phosphorylated form or the dephosphorylated form? Explain your answer.
(Essay)
4.8/5  (40)
(40)
Which of the following methods would be the most suitable to assess the relative purity of a protein in a sample you have prepared?
(Multiple Choice)
4.8/5  (38)
(38)
Globular proteins fold up into compact, spherical structures that have uneven surfaces.They tend to form multi-subunit complexes, which also have a rounded shape.Fibrous proteins, in contrast, span relatively large distances within the cell and in the extracellular space.Which of the proteins below is NOT classified as a fibrous protein?
(Multiple Choice)
4.7/5  (41)
(41)
For each of the following sentences, fill in the blanks with the best word or phrase selected from the list below.Not all words or phrases will be used; each word or phrase should be used only once.
allosteric ligand secondary domain primary subunit helix quaternary tertiary
The α helices and β sheets are examples of protein __________ structure.A protein such as hemoglobin, which is composed of more than one protein __________, has __________ structure.A protein's amino acid sequence is known as its __________ structure.A protein __________ is the modular unit from which many larger single-chain proteins are constructed.The three-dimensional conformation of a protein is its __________ structure.
(Essay)
4.8/5  (38)
(38)
One way in which an enzyme can lower the activation energy required for a reaction is to bind the substrate(s) and distort its structure so that the substrate more closely resembles the transition state of the reaction.This mechanism will be facilitated if the shape and chemical properties of the enzyme's active site are more complementary to the transition state than to the undistorted substrate; in other words, if the enzyme were to have a higher affinity for the transition state than for the substrate.Knowing this, your friend looked in an organic chemistry textbook to identify a stable chemical that closely resembles the transition state of a reaction that converts X into Y.She generated an antibody against this transition-state analog and mixed the antibody with chemical X.What do you think might happen?
(Essay)
4.8/5  (34)
(34)
Fully folded proteins typically have polar side chains on their surfaces, where electrostatic attractions and hydrogen bonds can form between the polar group on the amino acid and the polar molecules in the solvent.In contrast, some proteins have a polar side chain in their hydrophobic interior.Which of the following would NOT occur to help accommodate an internal, polar side chain?
(Multiple Choice)
4.9/5  (46)
(46)
Which of the following methods would be the most suitable to assess levels of expression of your target protein in different cell types?
(Multiple Choice)
4.7/5  (34)
(34)
To study how proteins fold, scientists must be able to purify the protein of interest, use solvents to denature the folded protein, and observe the process of refolding at successive time points.What is the effect of the solvents used in the denaturation process?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 41 - 60 of 71
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)