Exam 9: Acids and Bases, Ph, and Buffers
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
What is the role of the kidney in maintaining acid-base homeostasis in the blood?
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
D
Select the choice that correctly states whether the substance is an acid or a base.
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
B
A blood sample has a pH of 7.42.What is the concentration of hydronium in the sample?
(Multiple Choice)
4.8/5  (34)
(34)
Each circle is a sample of an aqueous acidic or basic solution.Which solution contains a weak acid? 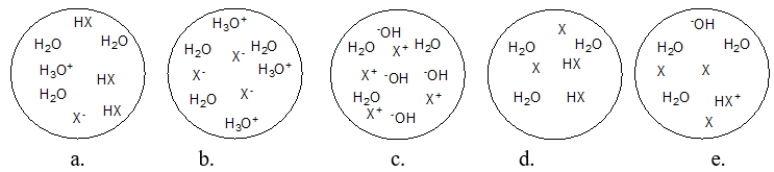
(Multiple Choice)
4.7/5  (35)
(35)
The reaction between acetic acid and water is given below, followed by a list of changes that could be made to the reaction solution.Which change will result in the equilibrium shifting to the left? CH3COOH + H2O ⇌ CH3COO- + H3O+
Changes that could be made to the solution
I.Adding more CH3COOH
II.Removing H2O
III.Removing H3O+
IV.Adding more CH3COO-
(Multiple Choice)
4.8/5  (39)
(39)
If the concentration of hydroxide ion in water is 1 × 10-4 M, the concentration of hydronium ion is ______ and there is more _____ in the solution.
(Multiple Choice)
4.8/5  (49)
(49)
What is the pH of a urine sample with a hydronium concentration of 7.9 × 10-8?
(Multiple Choice)
4.8/5  (34)
(34)
Each circle is a sample of an aqueous acidic or basic solution.Which solution contains a strong base? 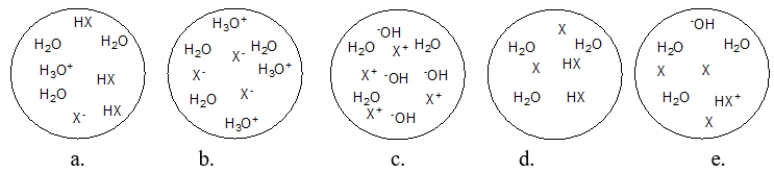
(Multiple Choice)
4.8/5  (33)
(33)
Adding an acid to pure water will change which of the following values: [H3O+], [OH-], and/or Kw?
(Multiple Choice)
4.7/5  (25)
(25)
The concentration of H3O+ in a solution is 1 × 10-4 M.What is the concentration of hydroxide ion in this solution?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following equations is the correctly balanced equation for the neutralization reaction of aluminum hydroxide and HCl?
(Multiple Choice)
4.8/5  (35)
(35)
Water can react as both an acid and a base, depending on its environment.Because of this characteristic, water is a(n)_________ molecule.
(Multiple Choice)
5.0/5  (38)
(38)
Which species in the following neutralization reaction are spectator ions? NaOH + HCl → H2O + NaCl
(Multiple Choice)
4.9/5  (34)
(34)
Showing 1 - 20 of 83
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)