Exam 9: Acids and Bases, Ph, and Buffers
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
If the concentration of hydronium ion in water is 1 × 10-12 M, the concentration of hydroxide ion is _____ and there is more _____ in the solution.
(Multiple Choice)
4.8/5  (39)
(39)
Which reaction BEST illustrates the reaction of an acid in aqueous solution?
(Multiple Choice)
4.7/5  (35)
(35)
What is the pH of a solution with a [OH- ] of 1.0 × 10-10 M?
(Multiple Choice)
4.7/5  (34)
(34)
The concentration of H3O+ in a solution is 1 × 10-4 M.Which statement describes how the concentration of OH- in the solution could be determined?
(Multiple Choice)
4.9/5  (49)
(49)
What mole ratio of NaOH to H2SO4 is needed in a neutralization reaction?
(Multiple Choice)
4.7/5  (28)
(28)
The following figure illustrates the action of a HF and F- buffer where the sizes of the boxes are proportional to the concentrations of HF and F- in solution.What will happen when a small amount of base (OH-)is added to the HF/F- buffer? 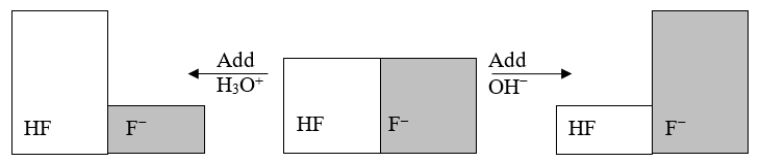
(Multiple Choice)
4.7/5  (38)
(38)
Consider a buffer solution containing CH3COO-Na+ and CH3COOH.If you add hydronium (H3O+)until all of the CH3COO- is converted into CH3COOH and then add a little more hydronium, what do you expect to observe?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following reactions illustrate the reaction of a base? I.NH3 + H2O → NH4+ + OH-
II)HCl + H2O → H3O+ + Cl-
III)NaOH → Na+ + OH-
IV)NH4+ + H2O → H3O+ + NH3
(Multiple Choice)
4.8/5  (38)
(38)
What is the pH of a solution with a [H3O+] of 7.9 × 10-11 M?
(Multiple Choice)
4.7/5  (38)
(38)
A weak acid is also a _________ because it produces a low concentration of ions in solution.
(Multiple Choice)
4.8/5  (38)
(38)
The neutralization reaction of potassium hydrogen carbonate (KHCO3)and HI produces what gas?
(Multiple Choice)
4.9/5  (38)
(38)
Which statement BEST describes what it means for the acid-base reaction between acetic acid and water to be in equilibrium? CH3COOH + H2O ⇌ CH3COO- + H3O+
(Multiple Choice)
4.7/5  (35)
(35)
Showing 61 - 80 of 83
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)