Exam 21: Heat Engines and Refrigerators
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions50 Questions
Exam 5: Force and Motion30 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics Ii: Motion in a Plane20 Questions
Exam 9: Work and Kinetic Energy66 Questions
Exam 10: Interactions and Potential Energy55 Questions
Exam 11: Impulse and Momentum43 Questions
Exam 12: Rotation of a Rigid Body116 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Fluids and Elasticity72 Questions
Exam 15: Oscillations49 Questions
Exam 16: Traveling Waves51 Questions
Exam 17: Superposition51 Questions
Exam 18: A Macroscopic Description of Matter46 Questions
Exam 19: Work, Heat, and the First Law of Thermodynamics96 Questions
Exam 20: The Micromacro Connection41 Questions
Exam 21: Heat Engines and Refrigerators44 Questions
Exam 22: Electric Charges and Forces26 Questions
Exam 23: The Electric Field32 Questions
Exam 24: Gausss Law41 Questions
Exam 25: The Electric Potential40 Questions
Exam 26: Potential and Field57 Questions
Exam 27: Current and Resistance32 Questions
Exam 28: Fundamentals of Circuits68 Questions
Exam 29: The Magnetic Field83 Questions
Exam 30: Electromagnetic Induction66 Questions
Exam 31: Electromagnetic Fields and Waves52 Questions
Exam 32: Ac Circuits44 Questions
Exam 33: Wave Optics51 Questions
Exam 34: Ray Optics60 Questions
Exam 35: Optical Instruments52 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics7 Questions
Exam 38: Quantization45 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics41 Questions
Exam 42: Nuclear Physics65 Questions
Select questions type
A Carnot refrigerator has a coefficient of performance of 2.5. The refrigerator consumes 50 W of power. How much heat is removed from the interior of the refrigerator in 1 hour?
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
B
A refrigerator has a coefficient of performance of 1.15, and it extracts 7.95 J of heat from the cold reservoir during each cycle.
(a) How much work is done on the gas in each cycle?
(b) How much heat is exhausted into the hot reservoir in each cycle?
Free
(Short Answer)
4.8/5  (40)
(40)
Correct Answer:
(a) 6.91 J (b) 14.9 J
A heat engine with an efficiency of 30.0% performs 2500 J of work. How much heat is discharged to the lower temperature reservoir?
Free
(Multiple Choice)
4.8/5  (43)
(43)
Correct Answer:
A
A Carnot engine operates between a high temperature reservoir at 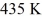 and a river with water at
and a river with water at 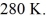 If it absorbs
If it absorbs 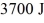 of heat each cycle, how much work per cycle does it perform?
of heat each cycle, how much work per cycle does it perform?
(Multiple Choice)
4.8/5  (37)
(37)
One of the most efficient engines built so far has the following characteristics:
combustion chamber temperature = 1900°C
exhaust temperature = 430°C
7.0 × 109 cal of fuel produces 1.4 × 1010 J of work in one hour, where 1 cal = 4.19 J
(a) What is the actual efficiency of this engine?
(b) What is the Carnot efficiency of the engine?
(c) What is the power output of this engine?
(Short Answer)
4.9/5  (35)
(35)
An ideal Carnot engine operates between reservoirs having temperatures of 125°C and
-20°C. Each cycle the heat expelled by this engine is used to melt 30.0 g of ice at 0.00°C. The heat of fusion of water is 3.34 × 105 J/kg and the heat of vaporization of water is
2.25 × 106 J/kg.
(a) How much work does this engine do each cycle?
(b) How much heat per cycle does this engine absorb at the hot reservoir?
(Short Answer)
4.9/5  (27)
(27)
A refrigerator has a coefficient of performance equal to 4.2. How much work must be done on the refrigerator in order to remove 250 J of heat from the interior?
(Multiple Choice)
4.8/5  (33)
(33)
A brass rod, 75.0 cm long and having a cross-sectional area of 2.50 cm2, conducts heat from a large furnace at 375°C into a very large cold water bath at 10.0°C without losing any heat at the lateral surface of the rod. Steady state has been established, and the thermal conductivity of brass is 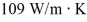 . The rate at which the entropy of the system (furnace plus water) is changing is closest to
. The rate at which the entropy of the system (furnace plus water) is changing is closest to
(Multiple Choice)
4.8/5  (28)
(28)
A Carnot cycle engine operates between a low temperature reservoir at 20°C and a high temperature reservoir at 800°C. If the engine is required to output 20.0 kJ of work per cycle, how much heat must the high temperature reservoir transfer to the engine during each cycle?
(Multiple Choice)
4.8/5  (30)
(30)
A real (non-Carnot) heat engine, operating between heat reservoirs at temperatures of 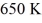 and
and 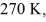 performs 4.3 kJ of net work and rejects
performs 4.3 kJ of net work and rejects 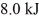 of heat in a single cycle. The thermal efficiency of this heat engine is closest to
of heat in a single cycle. The thermal efficiency of this heat engine is closest to
(Multiple Choice)
4.8/5  (32)
(32)
A certain engine extracts 1300 J of heat from a hot temperature reservoir and discharges 700 J of heat to a cold temperature reservoir. What is the efficiency of this engine?
(Multiple Choice)
4.9/5  (43)
(43)
What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 J/(mol ∙ K). 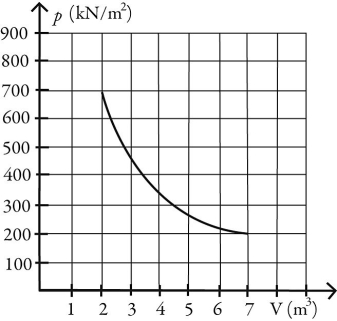
(Multiple Choice)
4.8/5  (29)
(29)
A refrigerator removes heat from the freezing compartment at the rate of 20 kJ and ejects 24 kJ into a room per cycle. How much work is required in each cycle?
(Multiple Choice)
5.0/5  (38)
(38)
A Carnot engine is operated as a heat pump to heat a room in the winter. The heat pump delivers heat to the room at the rate of 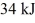 per second and maintains the room at a temperature of
per second and maintains the room at a temperature of 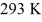 when the outside temperature is
when the outside temperature is 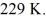 The power requirement for the heat pump under these operating conditions is closest to
The power requirement for the heat pump under these operating conditions is closest to
(Multiple Choice)
5.0/5  (32)
(32)
A Carnot air conditioner operates between an indoor temperature of 20°C and an outdoor temperature of 39°C. How much energy does it need to remove 2000 J of heat from the interior of the house?
(Multiple Choice)
5.0/5  (29)
(29)
What is the maximum theoretical efficiency possible for a heat engine operating between a reservoir in which ice and water coexist, and a reservoir in which water and steam coexist? The pressure is constant at 1.0 atmosphere for both reservoirs.
(Multiple Choice)
4.8/5  (34)
(34)
Is it possible to transfer heat from a hot reservoir to a cold reservoir?
(Multiple Choice)
4.8/5  (37)
(37)
A Carnot engine operating between a reservoir of liquid mercury at its melting point (233 K) and a colder reservoir extracts 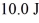 of heat from the mercury and does
of heat from the mercury and does 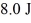 of work during each cycle. What is the temperature of the colder reservoir?
of work during each cycle. What is the temperature of the colder reservoir?
(Multiple Choice)
4.9/5  (40)
(40)
An air conditioner with a coefficient of performance of 3.5 uses 30 kW of power. How much power is it discharging to the outdoors?
(Multiple Choice)
4.7/5  (36)
(36)
During each cycle of operation, a refrigerator absorbs 230 J of heat from the freezer and expels 356 J of heat to the room. How much work input is required in each cycle?
(Multiple Choice)
4.9/5  (36)
(36)
Showing 1 - 20 of 44
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)