Exam 41: Atomic Physics
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions50 Questions
Exam 5: Force and Motion30 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics Ii: Motion in a Plane20 Questions
Exam 9: Work and Kinetic Energy66 Questions
Exam 10: Interactions and Potential Energy55 Questions
Exam 11: Impulse and Momentum43 Questions
Exam 12: Rotation of a Rigid Body116 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Fluids and Elasticity72 Questions
Exam 15: Oscillations49 Questions
Exam 16: Traveling Waves51 Questions
Exam 17: Superposition51 Questions
Exam 18: A Macroscopic Description of Matter46 Questions
Exam 19: Work, Heat, and the First Law of Thermodynamics96 Questions
Exam 20: The Micromacro Connection41 Questions
Exam 21: Heat Engines and Refrigerators44 Questions
Exam 22: Electric Charges and Forces26 Questions
Exam 23: The Electric Field32 Questions
Exam 24: Gausss Law41 Questions
Exam 25: The Electric Potential40 Questions
Exam 26: Potential and Field57 Questions
Exam 27: Current and Resistance32 Questions
Exam 28: Fundamentals of Circuits68 Questions
Exam 29: The Magnetic Field83 Questions
Exam 30: Electromagnetic Induction66 Questions
Exam 31: Electromagnetic Fields and Waves52 Questions
Exam 32: Ac Circuits44 Questions
Exam 33: Wave Optics51 Questions
Exam 34: Ray Optics60 Questions
Exam 35: Optical Instruments52 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics7 Questions
Exam 38: Quantization45 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics41 Questions
Exam 42: Nuclear Physics65 Questions
Select questions type
In a ruby laser, an electron jumps from a higher energy level to a lower one. If the energy difference between the two levels is 1.8 eV, what is the wavelength of the emitted photon? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
Free
(Multiple Choice)
4.9/5  (26)
(26)
Correct Answer:
D
A collection of atoms has 20% of the sample in a state 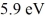 above the ground state. If these emit coherent radiation, what is the wavelength of the laser light produced? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
above the ground state. If these emit coherent radiation, what is the wavelength of the laser light produced? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
A
What is the correct electronic configuration for ground state carbon, which has 6 electrons?
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
A
What is the electron configuration for ground state Li, which has 3 electrons?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following are characteristics of laser light? (There may be more than one correct choice.)
(Multiple Choice)
4.7/5  (34)
(34)
If the principal quantum number of an electron is n = 5, which one of the following is NOT an allowed magnetic quantum number ml for the electron?
(Multiple Choice)
4.8/5  (30)
(30)
What is the minimum speed needed by a ground-state hydrogen atom for its kinetic energy to be enough to ionize the atom in a collision? (1 eV = 1.60 × 10-19 J, mh ≈ mproton = 1.67 × 10-27 kg)
(Multiple Choice)
4.7/5  (39)
(39)
An electron in a hydrogen atom has orbital quantum number l = 7. How many possible values of the magnetic quantum number ml could it have?
(Multiple Choice)
4.9/5  (39)
(39)
An electron in a hydrogen atom has orbital quantum number l = 4. How many possible values of the magnetic quantum number ml could it have?
(Multiple Choice)
4.9/5  (42)
(42)
If two electrons in the same atom have the same four quantum numbers, then they must have the same energy.
(Multiple Choice)
4.9/5  (30)
(30)
An atom with 5 electrons is in its ground state. How many electrons are in its outermost shell?
(Short Answer)
4.8/5  (32)
(32)
Consider the n = 9 shell.
(a) What is the largest value of the orbital quantum number, l, in this shell?
(b) How many electrons can be placed in this shell?
(Short Answer)
4.9/5  (37)
(37)
What is the energy of an incident photon that is just enough to excite a hydrogen atom from its ground state to its n = 4 state?
(Multiple Choice)
4.9/5  (36)
(36)
A neutral atom has an electron configuration of 1s22s22p63s23p2. How many protons does it have in its nucleus?
(Multiple Choice)
4.9/5  (29)
(29)
An electron in a hydrogen atom has principal quantum number n = 4. How many possible values of the orbital quantum number l could it have?
(Multiple Choice)
4.8/5  (39)
(39)
The normalized wave function for a hydrogen atom in the 1s state is given by ψ(r) = 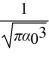 e-r/α0 where α0 is the Bohr radius, which is equal to 5.29 × 10-11 m. What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
e-r/α0 where α0 is the Bohr radius, which is equal to 5.29 × 10-11 m. What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
(Multiple Choice)
4.7/5  (23)
(23)
If two electrons in an atom have the same energy, then they must have the same four quantum numbers.
(Multiple Choice)
4.8/5  (34)
(34)
If the orbital quantum number is l = 4, which one of the following is a possible value for the principal quantum number n?
(Multiple Choice)
4.8/5  (32)
(32)
The correct ground state electron configuration of boron, which has 5 electrons, is
(Multiple Choice)
4.8/5  (25)
(25)
Showing 1 - 20 of 41
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)