Exam 38: Quantization
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions50 Questions
Exam 5: Force and Motion30 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics Ii: Motion in a Plane20 Questions
Exam 9: Work and Kinetic Energy66 Questions
Exam 10: Interactions and Potential Energy55 Questions
Exam 11: Impulse and Momentum43 Questions
Exam 12: Rotation of a Rigid Body116 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Fluids and Elasticity72 Questions
Exam 15: Oscillations49 Questions
Exam 16: Traveling Waves51 Questions
Exam 17: Superposition51 Questions
Exam 18: A Macroscopic Description of Matter46 Questions
Exam 19: Work, Heat, and the First Law of Thermodynamics96 Questions
Exam 20: The Micromacro Connection41 Questions
Exam 21: Heat Engines and Refrigerators44 Questions
Exam 22: Electric Charges and Forces26 Questions
Exam 23: The Electric Field32 Questions
Exam 24: Gausss Law41 Questions
Exam 25: The Electric Potential40 Questions
Exam 26: Potential and Field57 Questions
Exam 27: Current and Resistance32 Questions
Exam 28: Fundamentals of Circuits68 Questions
Exam 29: The Magnetic Field83 Questions
Exam 30: Electromagnetic Induction66 Questions
Exam 31: Electromagnetic Fields and Waves52 Questions
Exam 32: Ac Circuits44 Questions
Exam 33: Wave Optics51 Questions
Exam 34: Ray Optics60 Questions
Exam 35: Optical Instruments52 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics7 Questions
Exam 38: Quantization45 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics41 Questions
Exam 42: Nuclear Physics65 Questions
Select questions type
A laser emits light of wavelength 463 nm during a brief pulse that lasts for 25 ms and has a total energy of 1.2 J. How many photons are emitted in that single pulse? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s)
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
A
A metal having a work function of 2.8 eV is illuminated with monochromatic light whose photon energy is 3.9 eV. What is the threshold frequency for photoelectron production? (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
A
In a particular case of Compton scattering, a photon collides with a free electron and scatters backwards. The wavelength after the collision is exactly double the wavelength before the collision. What is the wavelength of the incident photon? (mel = 9.11 × 10-31 kg, h = 6.626 × 10-34 J ∙ s, c = 3.00 × 108 m/s)
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
B
X-rays of energy 2.9 × 104 eV are scattered by a free stationary electron through an angle of 135°. What is the energy of the scattered X-rays, in electron volts? (mel = 9.11 × 10-31 kg,
e = - 1.60 × 10-19 C, h = 6.626 × 10-34 J ∙ s, c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J)
(Short Answer)
4.9/5  (25)
(25)
A hydrogen atom initially in the n = 6 state decays to the n = 2 state. The emitted photon is detected in a photographic plate. What is the wavelength of the detected photon? The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J, c = 3.00 × 108 m/s)
(Multiple Choice)
4.9/5  (35)
(35)
A beam of red light and a beam of violet light each deliver the same power on a surface. For which beam is the number of photons hitting the surface per second the greatest?
(Multiple Choice)
5.0/5  (37)
(37)
What is the orbital radius of the 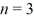 excited state in the Bohr model of the hydrogen atom? The ground-state radius of the hydrogen atom is 0.529 × 10-10 m.
excited state in the Bohr model of the hydrogen atom? The ground-state radius of the hydrogen atom is 0.529 × 10-10 m.
(Multiple Choice)
4.9/5  (35)
(35)
A photon of initial wavelength 0.651 nm, after being scattered from a free electron at rest, moves off at an angle of 120° with respect to its incident direction. (mel = 9.11 × 10-31 kg,
h = 6.626 × 10-34 J ∙ s, c = 3.00 × 108 m/s)
(a) What is the wavelength of the scattered photon?
(b) What is the energy of the scattered photon?
(Essay)
4.7/5  (40)
(40)
A hydrogen atom makes a downward transition from the 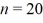 state to the n = 5 state. Find the wavelength of the emitted photon. The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J, c = 3.00 × 108 m/s)
state to the n = 5 state. Find the wavelength of the emitted photon. The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J, c = 3.00 × 108 m/s)
(Multiple Choice)
4.9/5  (35)
(35)
In a photoelectric effect experiment, electrons emerge from a copper surface with a maximum kinetic energy of 1.10 eV when light shines on the surface. The work function of copper is 4.65 eV. Which one of the following values is closest to the wavelength of the light?
(h = 6.626 × 10-34 J ∙ s, c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.9/5  (39)
(39)
A nonrelativistic electron is accelerated from rest through a potential difference. After acceleration the electron has a de Broglie wavelength of 880 nm. What is the potential difference though which this electron was accelerated? (h = 6.626 × 10-34 J ∙ s, e = -1.60 × 10-19 C, mel = 9.11 × 10-31 kg)
(Multiple Choice)
5.0/5  (36)
(36)
Light of wavelength 400 nm falls on a metal surface having a work function 1.70 eV. What is the maximum kinetic energy of the photoelectrons emitted from the metal? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s = 4.141 × 10-15 ev ∙ s, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.9/5  (42)
(42)
A gas of helium atoms (each of mass 6.65 × 10-27 kg) are at room temperature of 20.0°C. What is the de Broglie wavelength of the helium atoms that are moving at the root-mean-square speed? (h = 6.626 × 10-34 J ∙ s, the Boltzmann constant is 1.38 × 10-23 J/K)
(Multiple Choice)
4.8/5  (39)
(39)
A hydrogen atom is in its n = 2 excited state when its electron absorbs a photon of energy 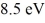 . What is the energy of the resulting free electron? The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
. What is the energy of the resulting free electron? The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.8/5  (37)
(37)
A photon of wavelength 29 pm is scattered by a stationary electron. What is the maximum possible energy loss of the photon? (mel = 9.11 × 10-31 kg, h = 6.626 × 10-34 J ∙ s, c = 3.00 × 108 m/s)
(Multiple Choice)
4.7/5  (41)
(41)
A single slit is illuminated at normal incidence with a parallel beam of light having a wavelength of 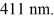 The entire central band of the diffraction pattern is observed at ±90°. The illumination is now replaced by a nonrelativistic beam of electrons, each having a kinetic energy of 980 eV. When this beam hits the slit at normal incidence, at what angle will the first minimum of the electron diffraction pattern occur? (h = 6.626 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg, 1 eV = 1.60 × 10-19 J)
The entire central band of the diffraction pattern is observed at ±90°. The illumination is now replaced by a nonrelativistic beam of electrons, each having a kinetic energy of 980 eV. When this beam hits the slit at normal incidence, at what angle will the first minimum of the electron diffraction pattern occur? (h = 6.626 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.8/5  (39)
(39)
How fast must a nonrelativistic electron move so its de Broglie wavelength is the same as the wavelength of a 3.4-eV photon? (mel = 9.11 × 10-31 kg, c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.8/5  (30)
(30)
A metal surface has a work function of 1.50 eV. Calculate the maximum kinetic energy, in eV, of electrons ejected from this surface by electromagnetic radiation of wavelength 311 nm.
(c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, e = -1.60 × 10-19 C, 1 eV = 1.60 × 10-19 J)
(Short Answer)
4.7/5  (40)
(40)
An 84-kW AM radio station broadcasts at 1000 kHz. How many photons are emitted each second by the transmitting antenna? (h = 6.626 × 10-34 J ∙ s)
(Multiple Choice)
4.9/5  (30)
(30)
Suppose that in a parallel universe, the proton and electron were identical to their counterparts in our own universe EXCEPT that the electron had twice as much charge as our electron. In our present universe, the radius of the first Bohr orbit for hydrogen is a0 and the speed of an electron in that orbit is v0. In the parallel universe,
(a) what would be the radius (in terms of a0) of the first Bohr orbit for hydrogen?
(b) what would be the speed (in terms of v0) of an electron in the first Bohr orbit for hydrogen?
(Essay)
4.9/5  (37)
(37)
Showing 1 - 20 of 45
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)