Exam 2: Essential Chemistry for Biology
Exam 1: Learning About Life45 Questions
Exam 2: Essential Chemistry for Biology50 Questions
Exam 3: The Molecules of Life56 Questions
Exam 4: A Tour of the Cell57 Questions
Exam 5: The Working Cell58 Questions
Exam 6: Cellular Respiration: Obtaining Energy From Food53 Questions
Exam 7: Photosynthesis: Using Light to Make Food52 Questions
Exam 8: Cellular Reproduction: Cells From Cells59 Questions
Exam 9: Patterns of Inheritance55 Questions
Exam 10: The Structure and Function of Dna59 Questions
Exam 11: How Genes Are Controlled55 Questions
Exam 12: Dna Technology54 Questions
Exam 13: How Populations Evolve52 Questions
Exam 14: How Biological Diversity Evolves47 Questions
Exam 15: The Evolution of Microbial Life59 Questions
Exam 16: The Evolution of Plants and Fungi56 Questions
Exam 17: The Evolution of Animals60 Questions
Exam 18: An Introduction to Ecology and the Biosphere56 Questions
Exam 19: Population Ecology52 Questions
Exam 20: Communities and Ecosystems63 Questions
Exam 21: Unifying Concepts of Animal Structure and Function57 Questions
Exam 22: Nutrition and Digestion67 Questions
Exam 23: Circulation and Respiration69 Questions
Exam 24: The Bodys Defenses59 Questions
Exam 25: Hormones53 Questions
Exam 26: Reproduction and Development59 Questions
Exam 27: Nervous, Sensory, and Locomotor Systems62 Questions
Exam 28: The Life of a Flowering Plant81 Questions
Exam 29: The Working Plant73 Questions
Select questions type
Consider the following reaction. What type of bond is holding the two atoms together? K + Br → K+ + Br− → KBr
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
B
The bond between oppositely charged ions is a(n)________ bond.
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
A
How can radiation be controlled and safely used in medicine?
(Multiple Choice)
4.9/5  (32)
(32)
A fossil was found and determined by radiometric dating to be 11,400 years old. What is the ratio of carbon-14 to carbon-12 in this fossil compared to its environment?
(Multiple Choice)
4.8/5  (30)
(30)
In order to have a positive charge, an atom must have ________.
(Multiple Choice)
4.9/5  (33)
(33)
Scenario
Please read the following scenario to answer the following questions.
The last few miles of the marathon are the most difficult for Heather. Her hair is plastered to her head, sweat clings to her arms, and her legs feel as if they had nothing left. Heather grabs a cup of ice water. The ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. Then a breeze kicks up and she finally feels some coolness against her skin. Drops of sweat, once clinging to her forehead, now spill down, and Heather feels a stinging as the sweat flows into her eyes.
-Why did the sweat on Heather's forehead and arms form drops?
(Multiple Choice)
4.9/5  (25)
(25)
What name is given to bonds that involve the sharing of electrons?
(Multiple Choice)
4.8/5  (35)
(35)
In the following figure, all of the representations EXCEPT one clearly show double bonds for the oxygen molecule. Choose the exception. 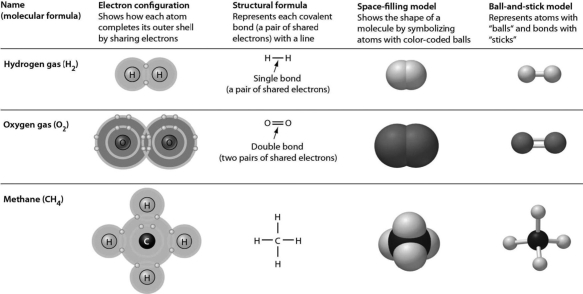
(Multiple Choice)
4.8/5  (33)
(33)
What type of cancer is treated with radioactive iodine seeds?
(Multiple Choice)
4.9/5  (30)
(30)
When a base is added to a buffered solution, the buffer will ________.
(Multiple Choice)
4.8/5  (36)
(36)
The tendency of molecules of the same kind to stick together is called ________.
(Multiple Choice)
4.7/5  (27)
(27)
Showing 1 - 20 of 50
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)