Exam 11: Solutions: Properties and Behavior
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
At 25°C, the vapor pressure of pure water is 25.756 mm Hg. What is the vapor pressure of water in a 0.500 m solution of sodium chloride?
(Multiple Choice)
4.7/5  (24)
(24)
Indicate which aqueous solution has the slowest evaporation rate.
(Multiple Choice)
4.9/5  (33)
(33)
Which of the solutions shown here will have the highest vapor pressure? White circles indicate solvent molecules; black circles indicate molecules of a nonvolatile solute.
(Multiple Choice)
5.0/5  (34)
(34)
Indicate which aqueous solution has the highest vapor pressure.
(Multiple Choice)
4.9/5  (41)
(41)
Which statement below regarding vapor pressure is not correct?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following gases do you expect to be most soluble in water?
(Multiple Choice)
4.9/5  (39)
(39)
The pressure of carbon dioxide in a beer bottle is about 2 atm. At this pressure about 0.15 g of carbon dioxide dissolves in 100 mL of beer. How much carbon dioxide remains dissolved in 100 mL after the bottle is opened? The partial pressure of carbon dioxide is 2.0 *10-4 atm.
(Multiple Choice)
5.0/5  (37)
(37)
What is the vapor pressure of an aqueous solution that has a solute mol fraction of 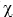 = 0.100? The vapor pressure of water is 25.756 mm Hg at 25°C.
= 0.100? The vapor pressure of water is 25.756 mm Hg at 25°C.
(Multiple Choice)
4.9/5  (39)
(39)
Why would you expect or predict sodium fluoride to have the lowest solubility in water compared to the other sodium halide salts?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following compounds has the largest melting point?
(Multiple Choice)
4.9/5  (37)
(37)
You are working as an intern in a biochemistry lab. Your advisor just isolated an enzyme and asks you to determine its molar mass using osmosis. You dissolve 1.0 g of the enzyme in water to make 250 mL of solution. You measure the osmotic pressure and find it to be 3.5 torr at 298 K. You report that the molar mass is ________ g/mol.
(Multiple Choice)
4.8/5  (45)
(45)
Determine the molal concentration of a sugar solution in water that has a freezing point of -2.1°C. Kf = 1.86°C/m for water.
(Multiple Choice)
5.0/5  (30)
(30)
Which intermolecular interactions are likely to result in the lowest vapor pressure of a substance?
(Multiple Choice)
4.9/5  (36)
(36)
The freezing point of a 0.0925 m solution of ammonium chloride was found to be -0.325°C. What is the actual van 't Hoff factor for this salt at this concentration compared to the ideal one of 2?
Kf (water) = 1.86°C/m
(Multiple Choice)
4.9/5  (41)
(41)
Which intermolecular interactions are likely to result in the highest vapor pressure of a substance?
(Multiple Choice)
4.8/5  (36)
(36)
Which statement about the vapor pressure of a liquid is not correct?
(Multiple Choice)
4.8/5  (26)
(26)
A 300 mg sample of caffeine was dissolved in 10.0 g of camphor (Kf = 39.7°C/m), decreasing the freezing point of camphor by 3.07°C. What is the molar mass of caffeine?
(Multiple Choice)
5.0/5  (34)
(34)
What is the molality of a solution produced by dissolving 14.40 g of LiCl (42.39 g/mol) in water to make 0.104 L of solution with a density of 1.102 g/mL?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following is not typically needed to calculate the lattice energy of an ionic compound?
(Multiple Choice)
4.9/5  (40)
(40)
Showing 21 - 40 of 130
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)