Exam 11: Solutions: Properties and Behavior
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Calculate the molality of a solution containing 0.755 mol glucose and 1.75 kg of water.
(Multiple Choice)
4.8/5  (26)
(26)
Which of the following will have the largest lattice energy?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following aqueous solutions will have the lowest freezing point?
(Multiple Choice)
4.8/5  (39)
(39)
Determine the energy change for the reaction
Li(s)+ Cl2(g) LiCl(s)
From the following data:
Lattice energy of LiCl = -861 kJ/mol
Energy to vaporize Li = 159 kJ/mol
Ionization energy of Li = 520 kJ/mol
Cl2 bond energy: 240 kJ/mol
Electron affinity of Cl: -349 kJ/mol
(Multiple Choice)
4.9/5  (33)
(33)
Which statement regarding the fractional distillation of two substances is not correct?
(Multiple Choice)
4.7/5  (25)
(25)
Which statement is not correct? Determination of molar mass of an unknown sample by an osmotic pressure measurement requires that ________
(Multiple Choice)
4.9/5  (45)
(45)
A solution contains 6.50 mol water, 0.300 mol sucrose, and 0.200 mol glucose. The solutes are nonvolatile. What is the vapor pressure of the solution at 35°C given that the vapor pressure of water is 42.2 torr?
(Multiple Choice)
4.9/5  (38)
(38)
Calculate the minimum pressure that must be applied to achieve reverse osmosis of 0.504 M NaCl at 22 C.
(Multiple Choice)
4.8/5  (29)
(29)
Caryophyllene, a nonelectrolyte, is one of the compounds responsible for the flavor of cloves. A 207 mg sample of caryophyllene was dissolved in 1.00 g of chloroform (Kb = 3.63°C/m), increasing the boiling point of chloroform by 3.68°C. What is the molar mass of caryophyllene?
(Multiple Choice)
4.9/5  (29)
(29)
Use the following data to calculate the enthalpy change for the following reaction: (s)+(g)\rightarrow(s) Quantity Magnitude (/) Ionization energy of (g) 425 Electron affinity of (g) -349 Vaporization energy of (s) 89 Bond energy of (g) 240 Lattice energy of (s) -723
(Multiple Choice)
4.7/5  (34)
(34)
You like boiled eggs for breakfast, but they take too long to cook, and you are always late to your early morning class. Lucky you! You have learned that adding table salt, NaCl (58.4 g/mol,
2)16 g/cm3), to water (Kb = 0.52°C/m) increases the temperature at which it boils. You figure that you can cook eggs faster at a higher temperature in boiling salty water! What increase in the boiling point do you expect if you add 1 tablespoon (1 tbsp = 14.8 cm3) of salt to one 8-oz cup of water (237 mL)?
(Multiple Choice)
4.7/5  (48)
(48)
A solution is made by dissolving 100 g of essentially nonvolatile ethylene glycol (C2H6O2) in
500 g of water. What is the resulting freezing point of the solution (Kf = 1.86°C/m)?
(Multiple Choice)
4.9/5  (30)
(30)
Which graph best describes how the vapor pressure of a solution varies according to Raoult's law as a nonvolatile solute is added to a liquid? The vapor pressure of the solution is plotted on the y-axis, and the mole fraction of solvent is plotted on the x-axis. The origin (0, 0) is not necessarily located where the axes cross. 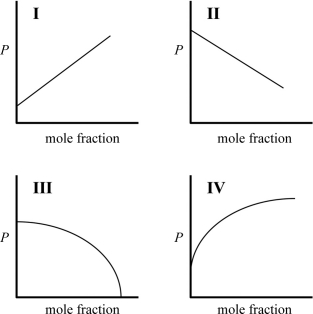
(Multiple Choice)
4.7/5  (34)
(34)
Indicate which aqueous solution has the fastest evaporation rate.
(Multiple Choice)
4.7/5  (43)
(43)
Which statement about how the vapor pressure (P) of a liquid depends on temperature is correct?
(Multiple Choice)
4.8/5  (43)
(43)
Describe how the molar mass of a compound can be determined from data obtained in two experiments: (1) measurements of the freezing point, and (2) measurements of the boiling point of a solution of the compound.
(Essay)
4.8/5  (37)
(37)
Which of the following ranks the compounds from lowest to highest lattice energy?
(Multiple Choice)
4.8/5  (44)
(44)
A physiological saline solution is 0.92% NaCl by mass. What is the osmotic pressure of such a solution at a body temperature of 37°C?
(Multiple Choice)
4.7/5  (32)
(32)
If water contains about 42 mg of oxygen per liter at 20°C and 1.0 atm, what would be the value of Henry's law constant for oxygen dissolving in water? The mole fraction of oxygen in air is 0.21.
(Multiple Choice)
4.7/5  (33)
(33)
Which graph best describes how the vapor pressure of a solution varies according to Raoult's law as a nonvolatile solute is added to a liquid? The vapor pressure of the solution is plotted on the y-axis, and the mole fraction of solute is plotted on the x-axis. The origin (0, 0) is not necessarily located where the axes cross. 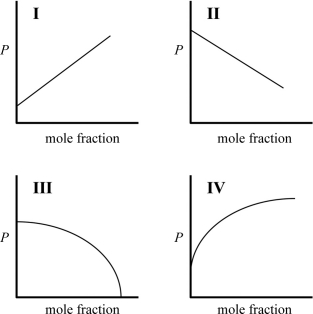
(Multiple Choice)
4.9/5  (37)
(37)
Showing 41 - 60 of 130
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)