Exam 11: Solutions: Properties and Behavior
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Determine the molarity of an aspirin solution that produces an osmotic pressure of 0.0555 atm at 25°C (i = 1).
(Short Answer)
4.8/5  (33)
(33)
Which one of the ionic compounds below would you expect to have the largest (most negative) lattice energy?
(Multiple Choice)
4.8/5  (42)
(42)
A 15.0 mg sample of a protein was dissolved in water to produce 5.00 mL of solution at 25.1°C. The osmotic pressure of this solution was measured and found to be 6.50 torr. What is the molar mass of this protein?
(Multiple Choice)
4.8/5  (33)
(33)
What would be the freezing point of a 1 m solution of HCl in water (Kf = 1.86°C)?
(Multiple Choice)
4.9/5  (30)
(30)
In the diagram below, one beaker contains pure water and the other contains an equal volume of seawater. Seawater has various salts dissolved in it. The beakers are sitting in a totally enclosed chamber, and the outside temperature and pressure are held constant. Identify the statements below about this situation that are not correct. 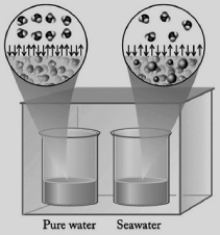 I. Water will be transferred from the pure water beaker to the seawater beaker.
II) The vapor pressure of the seawater is higher than the vapor pressure of the pure water.
III) Pure water evaporates at a faster rate than seawater.
IV) Water in the gas phase condenses into the seawater beaker at a faster rate than into the pure water beaker.
I. Water will be transferred from the pure water beaker to the seawater beaker.
II) The vapor pressure of the seawater is higher than the vapor pressure of the pure water.
III) Pure water evaporates at a faster rate than seawater.
IV) Water in the gas phase condenses into the seawater beaker at a faster rate than into the pure water beaker.
(Multiple Choice)
4.9/5  (42)
(42)
The solubility of any gas in a liquid can always be increased by ________
(Multiple Choice)
4.8/5  (26)
(26)
Which solution, if either, would create the higher osmotic pressure (compared to pure water): one prepared from 1.0 g of NaCl in 10 mL of water or 1.0 g of CsBr in 10 mL of water?
(Multiple Choice)
4.9/5  (34)
(34)
Use the following data to calculate the lattice energy of lithium oxide, Li2O. Quantity Magnitude (/) Ionization energy of (g) 519 Electron affinity of (g) for 2 603 Vaporization energy of (s) 147 Bond energy of (g) 499 Reaction enthalpy: 2(s)+(g)\rightarrow(s) -20
(Multiple Choice)
4.8/5  (35)
(35)
Ethylene glycol is used commonly as an antifreeze in automobile radiators. Automobile temperatures in the U.S. are commonly measured in degrees Fahrenheit. A 50:50 mixture of ethylene glycol and water by volume protects down to about -30°F. What is the boiling point in °F of this 50:50 mixture? (C2H6O2, 62.07 g/mol, 1.1132 g/mL, Kb = 0.94°F/m).
(Short Answer)
4.8/5  (40)
(40)
Which of the following requires the smallest energy to separate the ions?
(Multiple Choice)
4.9/5  (32)
(32)
A bottle is half-filled with a mixture of 25% liquid heptane and 75% liquid octane (mole fractions) at 25°C. What is the mole ratio of heptane to octane in the space above the liquid compared to in the liquid? The vapor pressures are 31 torr for pure heptane and 11 torr for pure octane.
(Essay)
4.8/5  (42)
(42)
A newspaper article suggested using a fertilizer such as ammonium sulfate or ammonium nitrate to lower the melting point of ice on sidewalks because many of the deicing salts can damage lawns and sidewalks. Which of the following compounds would give the largest freezing point depression when 100 g of the compound are dissolved in 1 kg of solvent?
(Multiple Choice)
4.9/5  (33)
(33)
Henry's law constant (mol/L · atm) for oxygen dissolving in blood is 3.74 *10-2 mol/L · atm at body temperature, 37°C. Calculate the molar concentration of oxygen in blood for an alpine climber where the atmospheric pressure is 0.45 atm. The mole fraction of oxygen in air is 0.209.
(Multiple Choice)
4.9/5  (30)
(30)
Describe how the data obtained by a measurement of osmotic pressure can be used to determine the molar mass of a compound.
(Essay)
4.8/5  (40)
(40)
Isopropyl alcohol has a boiling point of 82.3°C. Solutions of isopropyl alcohol in water have normal boiling points less than 100°C. Is this observation consistent with the following equation? Explain.
Tb = iKbm
(Multiple Choice)
4.9/5  (41)
(41)
The normal temperature range of the liquid phase of pure water is 0°C to 100°C. Which of the following solutions will have the largest temperature range for the liquid state?
(Multiple Choice)
4.8/5  (31)
(31)
In the process of dialysis, a special semipermeable membrane allows both small molecules and water to pass through, but not large protein molecules. These membranes are used to separate these small molecules and ions from much larger proteins. If a mixture of proteins and small molecules were separated from pure water by a dialysis membrane as shown in the figure, which way would the molecules flow? 
(Multiple Choice)
4.8/5  (36)
(36)
Showing 61 - 80 of 130
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)