Exam 26: Quantum Physics and Atomic Structure
Exam 1: Introduction to Physics60 Questions
Exam 2: Linear Motion84 Questions
Exam 3: Motion in Two and Three Dimensions94 Questions
Exam 4: Forces and Motion I: Newtons Laws93 Questions
Exam 5: Forces and Motion II: Applications75 Questions
Exam 6: Work and Energy85 Questions
Exam 7: Momentum, Collisions, and the Center of Mass75 Questions
Exam 8: Rotational Motion130 Questions
Exam 9: Elastic Properties of Matter: Stress and Strain49 Questions
Exam 10: Gravitation81 Questions
Exam 11: Fluids92 Questions
Exam 12: Oscillations124 Questions
Exam 13: Waves198 Questions
Exam 14: Thermodynamics I 146 Questions
Exam 15: Thermodynamics II120 Questions
Exam 16: Electrostatics I: Electric Charge, Forces, and Fields131 Questions
Exam 17: Electrostatics II: Electric Potential Energy and Electric Potential142 Questions
Exam 18: Electric Charges in Motion129 Questions
Exam 19: Magnetism105 Questions
Exam 20: Electromagnetic Induction50 Questions
Exam 21: Alternating-Current Circuits97 Questions
Exam 22: Electromagnetic Waves53 Questions
Exam 23: Wave Properties of Light182 Questions
Exam 24: Geometrical Optics120 Questions
Exam 25: Relativity86 Questions
Exam 26: Quantum Physics and Atomic Structure167 Questions
Exam 27: Nuclear Physics94 Questions
Exam 28: Particle Physics79 Questions
Select questions type
A photon of wavelength 80 nm is absorbed by the electron in the ground-state level of the hydrogen atom. Is this enough energy to ionize the atom? If so calculate the kinetic energy of the free electron.
(Multiple Choice)
4.8/5  (40)
(40)
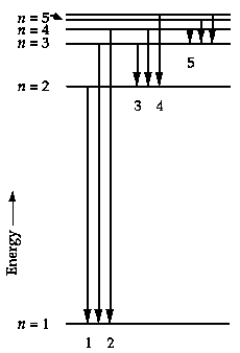 In the energy-level diagram, the line that corresponds to the longest wavelength in the Balmer series is
In the energy-level diagram, the line that corresponds to the longest wavelength in the Balmer series is
(Multiple Choice)
4.9/5  (37)
(37)
What is the energy difference between the transition with the longest wavelength and the transition with the shortest wavelength in the Balmer series?
(Multiple Choice)
4.9/5  (39)
(39)
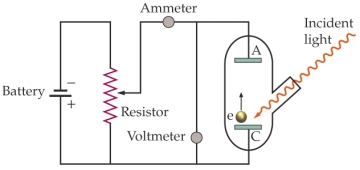 This is an apparatus for studying the photoelectric effect. The work function of the material being investigated is 3.8 * 10-19 J. Light of a wavelength 350 nm is incident on the material. What is the lowest voltage needed between the cathode C) and the anode A) to stop any electrons ejected from the cathode from reaching the anode?
This is an apparatus for studying the photoelectric effect. The work function of the material being investigated is 3.8 * 10-19 J. Light of a wavelength 350 nm is incident on the material. What is the lowest voltage needed between the cathode C) and the anode A) to stop any electrons ejected from the cathode from reaching the anode?
(Multiple Choice)
4.7/5  (32)
(32)
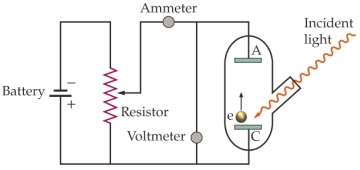 This is an apparatus for studying the photoelectric effect. The work function of the material being investigated is 3.5 *10-19 J. The battery is set at 1.5 V. What is the longest wavelength of light needed to produce an electric current from the cathode C) to the anode A)?
This is an apparatus for studying the photoelectric effect. The work function of the material being investigated is 3.5 *10-19 J. The battery is set at 1.5 V. What is the longest wavelength of light needed to produce an electric current from the cathode C) to the anode A)?
(Multiple Choice)
4.8/5  (34)
(34)
The order-of-magnitude of the diameter of the nucleus is closest to
(Multiple Choice)
4.9/5  (45)
(45)
Light of wavelength 400 nm is incident on a certain metal. The stopping potential for the emitted electrons is measured to be 1.2 V. What is the work function of this metal? Planck's constant h = 6.626 × 10-34 J · s = 4.136 × 10-15 eV · s.)
(Multiple Choice)
4.7/5  (42)
(42)
The number of electrons in the L shell for the element whose electronic configuration is 1s22s22p63s23p2 must be
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following statements concerning particles and waves is/are correct?
(Multiple Choice)
4.8/5  (43)
(43)
The maximum wavelength for the photoemission of electrons from a metal surface depends on
(Multiple Choice)
4.9/5  (36)
(36)
The wavelength of the visible line in the hydrogen spectrum that corresponds to m = 4 in the Balmer equation is
(Multiple Choice)
4.7/5  (45)
(45)
The energy of an electron in an atom is determined primarily by the quantum numbers
(Multiple Choice)
4.8/5  (31)
(31)
The photoelectric threshold of a certain metal is 310 nm. The maximum kinetic energy of electrons ejected from the surface of the metal by ultraviolet light of wavelength 200 nm is
(Multiple Choice)
4.9/5  (31)
(31)
If the work function of thoriated tungsten is 4 *10-19 J, the longest wavelength of light that will cause photoelectrons to be emitted is approximately
(Multiple Choice)
4.7/5  (33)
(33)
Showing 41 - 60 of 167
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)