Exam 26: Quantum Physics and Atomic Structure
Exam 1: Introduction to Physics60 Questions
Exam 2: Linear Motion84 Questions
Exam 3: Motion in Two and Three Dimensions94 Questions
Exam 4: Forces and Motion I: Newtons Laws93 Questions
Exam 5: Forces and Motion II: Applications75 Questions
Exam 6: Work and Energy85 Questions
Exam 7: Momentum, Collisions, and the Center of Mass75 Questions
Exam 8: Rotational Motion130 Questions
Exam 9: Elastic Properties of Matter: Stress and Strain49 Questions
Exam 10: Gravitation81 Questions
Exam 11: Fluids92 Questions
Exam 12: Oscillations124 Questions
Exam 13: Waves198 Questions
Exam 14: Thermodynamics I 146 Questions
Exam 15: Thermodynamics II120 Questions
Exam 16: Electrostatics I: Electric Charge, Forces, and Fields131 Questions
Exam 17: Electrostatics II: Electric Potential Energy and Electric Potential142 Questions
Exam 18: Electric Charges in Motion129 Questions
Exam 19: Magnetism105 Questions
Exam 20: Electromagnetic Induction50 Questions
Exam 21: Alternating-Current Circuits97 Questions
Exam 22: Electromagnetic Waves53 Questions
Exam 23: Wave Properties of Light182 Questions
Exam 24: Geometrical Optics120 Questions
Exam 25: Relativity86 Questions
Exam 26: Quantum Physics and Atomic Structure167 Questions
Exam 27: Nuclear Physics94 Questions
Exam 28: Particle Physics79 Questions
Select questions type
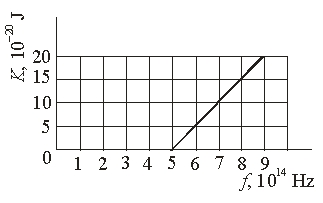 The graph shows the kinetic energy of photoelectrons ejected from a certain metal as a function of the frequency of the light incident upon the metal. The work function of this metal is
The graph shows the kinetic energy of photoelectrons ejected from a certain metal as a function of the frequency of the light incident upon the metal. The work function of this metal is
(Multiple Choice)
4.7/5  (31)
(31)
The energy of an x-ray photon of wavelength 3.0 × 10-10 m is
(Multiple Choice)
4.9/5  (47)
(47)
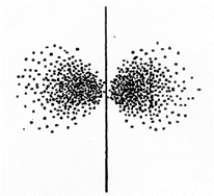 The set of quantum numbers for the probability density shown is
The set of quantum numbers for the probability density shown is
(Multiple Choice)
4.8/5  (35)
(35)
Light falling on the surface of a metal such as cesium can liberate electrons from the metal. The kinetic energy of electrons emitted from a metal can be increased by
(Multiple Choice)
4.8/5  (29)
(29)
The radii of the Bohr orbits in atomic hydrogen are given by 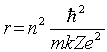 .If the radius of the first Bohr orbit n = 1) is 0.053 nm, the radius of the third Bohr orbit n = 3) is
.If the radius of the first Bohr orbit n = 1) is 0.053 nm, the radius of the third Bohr orbit n = 3) is
(Multiple Choice)
4.8/5  (42)
(42)
The red line in the hydrogen emission spectrum is 656 nm. If the energy of the nth level is -13.6/n 2 eV, then calculate the transition between n levels that this emitted photon comes from.
(Multiple Choice)
4.8/5  (33)
(33)
For an electron to have a de Broglie wavelength of 0.10 nm it must be accelerated from rest through a potential difference of
(Multiple Choice)
4.7/5  (38)
(38)
The radius of the n = 1 Bohr orbit in the hydrogen atom is 0.053 nm. What is the radius of the n = 5 Bohr orbit?
(Multiple Choice)
4.8/5  (33)
(33)
The energy of an electron in the n = 5 state in a hydrogen atom is approximately
(Multiple Choice)
4.9/5  (37)
(37)
Calculate the photon energy for light of wavelength λ = 500 nm.Planck's constant h = 6.626 × 10-34 J·s.)
(Multiple Choice)
4.7/5  (40)
(40)
In the Bohr model of the hydrogen atom, what is the kinetic energy of the electron in the n = 3 orbit? The radius of the first Bohr orbit is 0.0529 nm.)
(Multiple Choice)
4.8/5  (30)
(30)
When a certain x ray is Compton scattered at right angles to its initial direction, the shift in its wavelength is λ c = 2.4 pm 1 picometer = 10-12 m). If the wavelength of this ray is 15.4 pm, the wavelength of the scattered x ray must be closest to
(Multiple Choice)
4.8/5  (27)
(27)
The wavelength of the photon emitted when a hydrogen atom undergoes a transition from the n = 10 state to the n = 1 state is approximately
(Multiple Choice)
4.9/5  (32)
(32)
Potassium has a work function of 2.3 eV for photoelectric emission. Which of the following wavelengths is the longest wavelength for which photoemission occurs?
(Multiple Choice)
4.9/5  (37)
(37)
According to the Bohr theory, the allowed energy states for the hydrogen atom are given by the relation 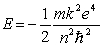 .This formula can be readily extended to other hydrogenic one-electron) systems. The energy of the second level n = 2) for the doubly ionized lithium atom is
.This formula can be readily extended to other hydrogenic one-electron) systems. The energy of the second level n = 2) for the doubly ionized lithium atom is
(Multiple Choice)
4.7/5  (36)
(36)
In a photoelectric experiment, the threshold frequency for a material is 3.2 × 1014 Hz. An electron ejected from this surface by a photon of frequency 9.4 × 1014 Hz can be stopped by a stopping potential of
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following experiments) illustrates the particle nature of light?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 141 - 160 of 167
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)