Exam 26: Quantum Physics and Atomic Structure
Exam 1: Introduction to Physics60 Questions
Exam 2: Linear Motion84 Questions
Exam 3: Motion in Two and Three Dimensions94 Questions
Exam 4: Forces and Motion I: Newtons Laws93 Questions
Exam 5: Forces and Motion II: Applications75 Questions
Exam 6: Work and Energy85 Questions
Exam 7: Momentum, Collisions, and the Center of Mass75 Questions
Exam 8: Rotational Motion130 Questions
Exam 9: Elastic Properties of Matter: Stress and Strain49 Questions
Exam 10: Gravitation81 Questions
Exam 11: Fluids92 Questions
Exam 12: Oscillations124 Questions
Exam 13: Waves198 Questions
Exam 14: Thermodynamics I 146 Questions
Exam 15: Thermodynamics II120 Questions
Exam 16: Electrostatics I: Electric Charge, Forces, and Fields131 Questions
Exam 17: Electrostatics II: Electric Potential Energy and Electric Potential142 Questions
Exam 18: Electric Charges in Motion129 Questions
Exam 19: Magnetism105 Questions
Exam 20: Electromagnetic Induction50 Questions
Exam 21: Alternating-Current Circuits97 Questions
Exam 22: Electromagnetic Waves53 Questions
Exam 23: Wave Properties of Light182 Questions
Exam 24: Geometrical Optics120 Questions
Exam 25: Relativity86 Questions
Exam 26: Quantum Physics and Atomic Structure167 Questions
Exam 27: Nuclear Physics94 Questions
Exam 28: Particle Physics79 Questions
Select questions type
A compact disc of a CD player has a moment of inertia I = 2.5 × 10-5 kg · m2 and rotates at 500 rev/min. Taking L = Iω and using the quantization of angular momentum, the approximate value of n is 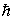 = 1.055 × 10-34 J · s)
= 1.055 × 10-34 J · s)
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following formulas has the correct units for the linear momentum of a photon?
(Multiple Choice)
4.8/5  (39)
(39)
The ground-state energy of hydrogen is -13.6 eV. The difference in energy between the n = 3 and n = 4 levels magnitude only) is
(Multiple Choice)
4.8/5  (40)
(40)
The equation derived by Bohr for the wavelengths of λ of the lines in hydrogen- like spectra is 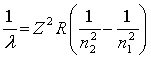 .The first member of the Balmer series of hydrogen has λ = 660 nm. Doubly ionized
.The first member of the Balmer series of hydrogen has λ = 660 nm. Doubly ionized 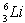 is hydrogen-like. The wavelength of the first member of the Balmer series for doubly ionized
is hydrogen-like. The wavelength of the first member of the Balmer series for doubly ionized 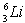 is
is
(Multiple Choice)
4.9/5  (36)
(36)
50 eV) when it is illuminated by light of wavelength 350 nm is
(Multiple Choice)
4.8/5  (44)
(44)
The energy of the n = 1 level of hydrogen is -13.6 eV. The energy of the n = 4 level is
(Multiple Choice)
4.9/5  (38)
(38)
Showing 161 - 167 of 167
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)