Exam 26: Quantum Physics and Atomic Structure
Exam 1: Introduction to Physics60 Questions
Exam 2: Linear Motion84 Questions
Exam 3: Motion in Two and Three Dimensions94 Questions
Exam 4: Forces and Motion I: Newtons Laws93 Questions
Exam 5: Forces and Motion II: Applications75 Questions
Exam 6: Work and Energy85 Questions
Exam 7: Momentum, Collisions, and the Center of Mass75 Questions
Exam 8: Rotational Motion130 Questions
Exam 9: Elastic Properties of Matter: Stress and Strain49 Questions
Exam 10: Gravitation81 Questions
Exam 11: Fluids92 Questions
Exam 12: Oscillations124 Questions
Exam 13: Waves198 Questions
Exam 14: Thermodynamics I 146 Questions
Exam 15: Thermodynamics II120 Questions
Exam 16: Electrostatics I: Electric Charge, Forces, and Fields131 Questions
Exam 17: Electrostatics II: Electric Potential Energy and Electric Potential142 Questions
Exam 18: Electric Charges in Motion129 Questions
Exam 19: Magnetism105 Questions
Exam 20: Electromagnetic Induction50 Questions
Exam 21: Alternating-Current Circuits97 Questions
Exam 22: Electromagnetic Waves53 Questions
Exam 23: Wave Properties of Light182 Questions
Exam 24: Geometrical Optics120 Questions
Exam 25: Relativity86 Questions
Exam 26: Quantum Physics and Atomic Structure167 Questions
Exam 27: Nuclear Physics94 Questions
Exam 28: Particle Physics79 Questions
Select questions type
According to Bohr's model, the radius of an electron orbit for n = 4 in a hydrogen atom is
(Multiple Choice)
4.9/5  (34)
(34)
If the frequency of light causing photoemission of electrons is doubled, the kinetic energy of the ejected electrons
(Multiple Choice)
4.9/5  (39)
(39)
A proton has five times the momentum of an electron. If the electron has a de Broglie wavelength λ, then the de Broglie wavelength of the proton is
(Multiple Choice)
4.9/5  (44)
(44)
The wave-particle duality theory is the first adequate explanation of which one of the following observations about the hydrogen atom?
(Multiple Choice)
4.8/5  (37)
(37)
The energy associated with the lowest n = 1 state in a hydrogen atom is - 13.6 eV. The wavelength of the emission line corresponding to the transition from n = 4 to n = 3 is
(Multiple Choice)
4.9/5  (33)
(33)
The electron microscope is a welcome addition to the field of microscopy because electrons have a __________ wavelength than light, thereby increasing the __________ of the microscope.
(Multiple Choice)
4.8/5  (44)
(44)
An electron in the hydrogen atom ground-state energy = -13.6 eV) makes a transition from the n = 3 to the n = 1 energy level. Calculate the magnitude of the energy of the photon involved in this process and state whether the photon was absorbed or emitted.
(Multiple Choice)
4.8/5  (37)
(37)
What is the momentum in SI units) of a photon of wavelength λ = 560 nm? Planck's constant h = 6.626 × 10-34 J·s.)
(Multiple Choice)
4.9/5  (42)
(42)
Calculate the photon energy for light of wavelength λ = 600 nm.Planck's constant h = 6.626 × 10-34 J·s.)
(Multiple Choice)
4.7/5  (35)
(35)
The energy of the nth level in a one-electron atom is 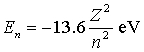 . Consider a beryllium ion with all but one of its electrons removed a beryllium atom normally has four electrons). What is the wavelength of a photon emitted when the electron makes the transition from the third-lowest to the lowest energy state?
. Consider a beryllium ion with all but one of its electrons removed a beryllium atom normally has four electrons). What is the wavelength of a photon emitted when the electron makes the transition from the third-lowest to the lowest energy state?
(Multiple Choice)
4.7/5  (33)
(33)
The first Bohr radius, a0, is 0.0529 nm and the corresponding energy, E0, is 13.6 eV. The wavelength of the light emitted as a hydrogen atom undergoes a transition from state n = 4 to n = 2 is
(Multiple Choice)
4.7/5  (46)
(46)
The energy of a quantum of radiation that has a wavelength of 447 nm in a vacuum is approximately
(Multiple Choice)
4.9/5  (35)
(35)
Magnetic resonance imaging MRI) is a much-used noninvasive diagnostic technique in medicine. The radio frequency photons used have energies of about 10-7 eV. What wavelength does this correspond to?
(Multiple Choice)
4.8/5  (37)
(37)
The constant in the Rydberg formula is RH = 10.96776 µm-1. The wavelength predicted by this formula for m = 3 and n = 2 is
(Multiple Choice)
4.9/5  (39)
(39)
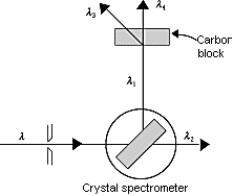 The figure shows x rays incident on a crystal spectrometer that are reflected from the crystal and used for Compton scattering in a carbon block. Of the wavelengths shown, λ3 ≠ λ; thus, which of the following expressions is true?
The figure shows x rays incident on a crystal spectrometer that are reflected from the crystal and used for Compton scattering in a carbon block. Of the wavelengths shown, λ3 ≠ λ; thus, which of the following expressions is true?
(Multiple Choice)
4.9/5  (27)
(27)
The critical experiments that established the nuclear nature of atoms were performed by
(Multiple Choice)
4.9/5  (29)
(29)
Estimate the number of photons emitted by the Sun in a second. The power output from the Sun is 4 × 1026 W and assume that the average wavelength of each photon is 550 nm.
(Multiple Choice)
4.8/5  (45)
(45)
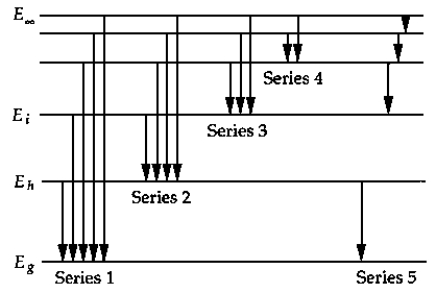 The above figure shows a schematic energy-level diagram for the hydrogen atom. The series that represents the Balmer series is
The above figure shows a schematic energy-level diagram for the hydrogen atom. The series that represents the Balmer series is
(Multiple Choice)
4.9/5  (33)
(33)
Showing 121 - 140 of 167
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)