Exam 26: Quantum Physics and Atomic Structure
Exam 1: Introduction to Physics60 Questions
Exam 2: Linear Motion84 Questions
Exam 3: Motion in Two and Three Dimensions94 Questions
Exam 4: Forces and Motion I: Newtons Laws93 Questions
Exam 5: Forces and Motion II: Applications75 Questions
Exam 6: Work and Energy85 Questions
Exam 7: Momentum, Collisions, and the Center of Mass75 Questions
Exam 8: Rotational Motion130 Questions
Exam 9: Elastic Properties of Matter: Stress and Strain49 Questions
Exam 10: Gravitation81 Questions
Exam 11: Fluids92 Questions
Exam 12: Oscillations124 Questions
Exam 13: Waves198 Questions
Exam 14: Thermodynamics I 146 Questions
Exam 15: Thermodynamics II120 Questions
Exam 16: Electrostatics I: Electric Charge, Forces, and Fields131 Questions
Exam 17: Electrostatics II: Electric Potential Energy and Electric Potential142 Questions
Exam 18: Electric Charges in Motion129 Questions
Exam 19: Magnetism105 Questions
Exam 20: Electromagnetic Induction50 Questions
Exam 21: Alternating-Current Circuits97 Questions
Exam 22: Electromagnetic Waves53 Questions
Exam 23: Wave Properties of Light182 Questions
Exam 24: Geometrical Optics120 Questions
Exam 25: Relativity86 Questions
Exam 26: Quantum Physics and Atomic Structure167 Questions
Exam 27: Nuclear Physics94 Questions
Exam 28: Particle Physics79 Questions
Select questions type
The quantum theory suggests that the stable orbits of electrons around a nucleus correspond to standing waves for the orbit. For a hydrogen atom, the radius of the ground state or the first orbit is the Bohr's radius, 0.0529 nm. What is the wavelength of the ground state of the hydrogen atom?
(Multiple Choice)
4.8/5  (28)
(28)
The first Bohr radius, a0, is 0.0529 nm and the corresponding energy, E0, is 13.6 eV. The wavelength of the light emitted as a hydrogen atom undergoes a transition from state n = 3 to n = 2 is about
(Multiple Choice)
4.7/5  (32)
(32)
The wavelength of the photon emitted when a hydrogen atom undergoes a transition from the n = 5 state to the n = 1 state is approximately
(Multiple Choice)
4.8/5  (32)
(32)
An electron in a hydrogen atom jumps from n = 5 to n = 2. The wavelength of the emitted photon is
(Multiple Choice)
4.9/5  (35)
(35)
In a Compton scattering experiment, an 8.00-MeV incident photon is scattered at an angle of 15º with energy of 5.20 MeV. The kinetic energy of the recoil electron is
(Multiple Choice)
4.9/5  (35)
(35)
The total number of distinct electron states including spin) when n = 5 is
(Multiple Choice)
4.8/5  (35)
(35)
For an x-ray tube to produce x rays with a wavelength as short as 0.025 nm, the electrons must be accelerated by a potential difference of
(Multiple Choice)
4.8/5  (29)
(29)
The number of oxygen's eight electrons that are found in the p state is
(Multiple Choice)
5.0/5  (40)
(40)
An electron in the n = 3 state of a hydrogen atom falls into the n = 2 state. The Rydberg constant R is 1.097 × 10-2/nm. The wavelength of the photon emitted is approximately
(Multiple Choice)
4.8/5  (38)
(38)
The work function for a metal is 5.58 eV. What is the threshold wavelength for the photoelectric effect of incident photons?
(Multiple Choice)
4.8/5  (37)
(37)
The constant in the Rydberg formula is RH = 10.96776 µm-1. The wavelength predicted by this formula for m = 5 and n = 3 is
(Multiple Choice)
4.9/5  (33)
(33)
An x-ray photon scattering off an electron imparts some of its energy to the electron the Compton effect). Which of the following statements is true?
(Multiple Choice)
4.9/5  (31)
(31)
Rutherford's experiments, in which he bombarded a very thin gold foil with alpha particles, showed that
(Multiple Choice)
4.7/5  (38)
(38)
 The set of quantum numbers for the probability density shown is
The set of quantum numbers for the probability density shown is
(Multiple Choice)
4.9/5  (35)
(35)
The order-of-magnitude of the diameter of an atom is closest to
(Multiple Choice)
4.8/5  (36)
(36)
For the principal quantum number n = 4, the number of values the orbital quantum number 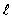 can have is
can have is
(Multiple Choice)
4.8/5  (41)
(41)
The possible orbital quantum numbers of an electron are 0, 1, 3, 4, and 5. Its principal quantum number, n, and the largest magnetic quantum number, ml, are
(Multiple Choice)
4.9/5  (33)
(33)
An electron in the hydrogen atom makes a transition from an energy level of -1.51 eV to one of energy -3.40 eV. In this process a photon is emitted with what wavelength?
(Multiple Choice)
4.9/5  (36)
(36)
A gamma-ray photon of energy 800 keV scatters off an electron at an angle perpendicular to its original direction. It then scatters off a second electron such that this secondary scattered photon continues in the direction of the original photon. Calculate the difference in energy between the first and second recoiling electrons.
(Multiple Choice)
4.7/5  (38)
(38)
Showing 81 - 100 of 167
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)