Exam 26: Quantum Physics and Atomic Structure
Exam 1: Introduction to Physics60 Questions
Exam 2: Linear Motion84 Questions
Exam 3: Motion in Two and Three Dimensions94 Questions
Exam 4: Forces and Motion I: Newtons Laws93 Questions
Exam 5: Forces and Motion II: Applications75 Questions
Exam 6: Work and Energy85 Questions
Exam 7: Momentum, Collisions, and the Center of Mass75 Questions
Exam 8: Rotational Motion130 Questions
Exam 9: Elastic Properties of Matter: Stress and Strain49 Questions
Exam 10: Gravitation81 Questions
Exam 11: Fluids92 Questions
Exam 12: Oscillations124 Questions
Exam 13: Waves198 Questions
Exam 14: Thermodynamics I 146 Questions
Exam 15: Thermodynamics II120 Questions
Exam 16: Electrostatics I: Electric Charge, Forces, and Fields131 Questions
Exam 17: Electrostatics II: Electric Potential Energy and Electric Potential142 Questions
Exam 18: Electric Charges in Motion129 Questions
Exam 19: Magnetism105 Questions
Exam 20: Electromagnetic Induction50 Questions
Exam 21: Alternating-Current Circuits97 Questions
Exam 22: Electromagnetic Waves53 Questions
Exam 23: Wave Properties of Light182 Questions
Exam 24: Geometrical Optics120 Questions
Exam 25: Relativity86 Questions
Exam 26: Quantum Physics and Atomic Structure167 Questions
Exam 27: Nuclear Physics94 Questions
Exam 28: Particle Physics79 Questions
Select questions type
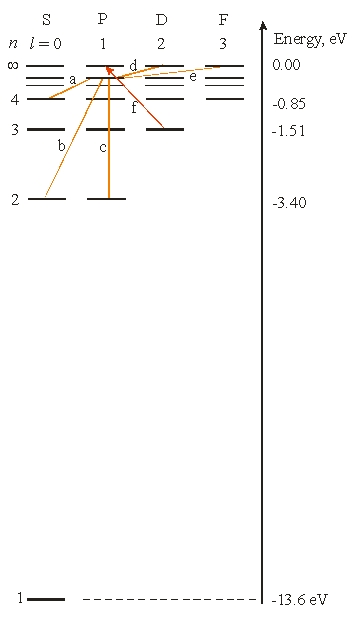 What is the wavelength of incident light needed for an electron to make the transition labeled f)?
What is the wavelength of incident light needed for an electron to make the transition labeled f)?
(Multiple Choice)
4.8/5  (37)
(37)
Z for the element whose electronic configuration is 1s22s22p63s23p2 must be
(Multiple Choice)
4.8/5  (38)
(38)
What is the shift in wavelength of a photon scattered off an electron at 160º?
(Multiple Choice)
4.7/5  (43)
(43)
The number of electrons in the M shell for the element whose electronic configuration is 1s22s22p63s23p2 must be
(Multiple Choice)
4.9/5  (39)
(39)
Electrons do not exhibit wave properties as readily as light because electrons typically have much __________ momenta than light and hence much __________ wavelengths.
(Multiple Choice)
4.9/5  (38)
(38)
An alpha particle mass = 4 amu) is moving twice as fast as a proton mass = 1 amu). Calculate the de Broglie wavelength of the proton divided by the de Broglie wavelength of the alpha particle.
(Multiple Choice)
4.9/5  (44)
(44)
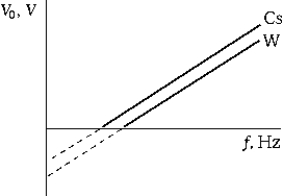 The graph shows the stopping potential for photoelectrons as a function of the frequency of the incident light for two different metals Cs and W). Which of the following statements is true?
The graph shows the stopping potential for photoelectrons as a function of the frequency of the incident light for two different metals Cs and W). Which of the following statements is true?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following statements about Maxwell's theory of electromagnetism is false?
(Multiple Choice)
4.8/5  (26)
(26)
Calculate the wavelength of light of energy 1 keV divided by the de Broglie wavelength of a proton of kinetic energy 1 meV.
(Multiple Choice)
4.8/5  (39)
(39)
An electron in the hydrogen atom ground-state energy = -13.6 eV) makes a transition from the n = 2 to the n = 4 energy level. Calculate the magnitude of the energy of the photon involved in this process and state whether the photon was absorbed or emitted.
(Multiple Choice)
4.9/5  (39)
(39)
When a surface is illuminated with light of wavelength 500 nm, the maximum speed of the photoelectrons is 300 km/s. When the same surface is illuminated by light of half this wavelength, what is the maximum speed of the photoelectrons in this case?
(Multiple Choice)
4.9/5  (34)
(34)
What is the ratio of the radius of the n = 3 orbit to that of the n = 2 orbit?
(Multiple Choice)
4.8/5  (27)
(27)
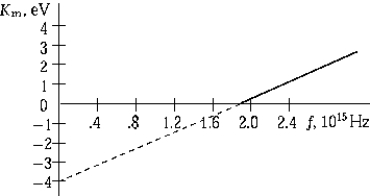 The graph shows the maximum kinetic energy of electrons emitted by a photosensitive surface as a function of the frequency of the incident radiation. The slope of this curve represents
The graph shows the maximum kinetic energy of electrons emitted by a photosensitive surface as a function of the frequency of the incident radiation. The slope of this curve represents
(Multiple Choice)
4.9/5  (39)
(39)
Showing 61 - 80 of 167
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)