Exam 3: Atomic Structure Explaining the Properties of Elements
Exam 1: Matter and Energy an Atomic Perspective138 Questions
Exam 2: Atoms, Ions, and Molecules the Building Blocks of Matter143 Questions
Exam 3: Atomic Structure Explaining the Properties of Elements175 Questions
Exam 4: Chemical Bonding Understanding Climate Change182 Questions
Exam 5: Bonding Theories Explaining Molecular Geometry141 Questions
Exam 6: Intermolecular Forces Attractions Between Particles87 Questions
Exam 7: Stoichiometry Mass Relationships and Chemical Reactions140 Questions
Exam 8: Aqueous Solutions Chemistry of the Hydrosphere180 Questions
Exam 9: Thermochemistry Energy Changes in Chemical Reactions215 Questions
Exam 10: Properties of Gases the Air We Breathe164 Questions
Exam 11: Properties of Solutions Their Concentrations and Colligative Properties130 Questions
Exam 12: Thermodynamics Why Chemical Reactions Happen130 Questions
Exam 13: Chemical Kinetics Clearing the Air172 Questions
Exam 14: Chemical Equilibrium Equal but Opposite Reaction Rates119 Questions
Exam 15: Acid-Base Equilibria Proton Transfer in Biological Systems123 Questions
Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans114 Questions
Exam 17: Electrochemistry the Quest for Clean Energy135 Questions
Exam 18: The Solid State a Particulate View170 Questions
Exam 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials145 Questions
Exam 20: Biochemistry the Compounds of Life153 Questions
Exam 21: Nuclear Chemistry the Risks and Benefits168 Questions
Exam 22: The Main Group Elements Life and the Periodic Table116 Questions
Exam 23: Transition Metals Biological and Medical Applications119 Questions
Select questions type
Which statement below about an electron shell in a hydrogen-like atom is FALSE?
(Multiple Choice)
4.8/5  (38)
(38)
Which row 2 element probably has the following successive ionization energies:
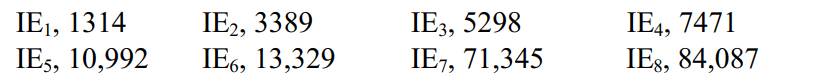
(Multiple Choice)
4.9/5  (39)
(39)
Astronomers have detected hydrogen atoms in interstellar space in the n = 732 energy level.Suppose an atom in this excited state emits a photon and undergoes a transition from n = 732 to n = 632.How much energy does the atom lose as a result of this transition? What is the frequency of this radiation? In which spectral region does this radiation lie?
(Essay)
4.7/5  (31)
(31)
Which of the following statements about electron spin and the spin quantum number, ms, is NOT true?
(Multiple Choice)
4.8/5  (38)
(38)
Which one of the following sources produces the highest energy photons?
(Multiple Choice)
4.9/5  (35)
(35)
When the principal quantum number = 3, what values are possible for the other quantum numbers and ml ?
(Essay)
5.0/5  (42)
(42)
What is the velocity of an O2 molecule if its de Broglie wavelength is 26 pm?
(1 amu = 1.66054 *10-27 kg.)
(Multiple Choice)
4.9/5  (37)
(37)
What are the principal and angular momentum quantum numbers for a 4 orbital?
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following elements would you expect to have the lowest first ionization energy?
(Multiple Choice)
4.8/5  (42)
(42)
Mercury lamps emit blue light with = 436 nm.What is the energy of these photons in joules?
(Multiple Choice)
4.7/5  (36)
(36)
Copper commonly forms a cation with a charge of 1+; however, the element to the right of it in the periodic table Zn) typically only forms a cation with a 2+charge.Use your knowledge of electron configurations to explain this observation.
(Essay)
4.8/5  (42)
(42)
What is the wavelength ( , in meters) of a radio station operating at a frequency of 99.6 MHz?
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following is the correct electron configuration for silicon?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following lasers emits photons with the lowest energy?
(Multiple Choice)
4.7/5  (41)
(41)
Which of the following shows the correct expression for the ionization energy indicated?
(Multiple Choice)
4.8/5  (32)
(32)
Showing 41 - 60 of 175
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)