Exam 3: Atomic Structure Explaining the Properties of Elements
Exam 1: Matter and Energy an Atomic Perspective138 Questions
Exam 2: Atoms, Ions, and Molecules the Building Blocks of Matter143 Questions
Exam 3: Atomic Structure Explaining the Properties of Elements175 Questions
Exam 4: Chemical Bonding Understanding Climate Change182 Questions
Exam 5: Bonding Theories Explaining Molecular Geometry141 Questions
Exam 6: Intermolecular Forces Attractions Between Particles87 Questions
Exam 7: Stoichiometry Mass Relationships and Chemical Reactions140 Questions
Exam 8: Aqueous Solutions Chemistry of the Hydrosphere180 Questions
Exam 9: Thermochemistry Energy Changes in Chemical Reactions215 Questions
Exam 10: Properties of Gases the Air We Breathe164 Questions
Exam 11: Properties of Solutions Their Concentrations and Colligative Properties130 Questions
Exam 12: Thermodynamics Why Chemical Reactions Happen130 Questions
Exam 13: Chemical Kinetics Clearing the Air172 Questions
Exam 14: Chemical Equilibrium Equal but Opposite Reaction Rates119 Questions
Exam 15: Acid-Base Equilibria Proton Transfer in Biological Systems123 Questions
Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans114 Questions
Exam 17: Electrochemistry the Quest for Clean Energy135 Questions
Exam 18: The Solid State a Particulate View170 Questions
Exam 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials145 Questions
Exam 20: Biochemistry the Compounds of Life153 Questions
Exam 21: Nuclear Chemistry the Risks and Benefits168 Questions
Exam 22: The Main Group Elements Life and the Periodic Table116 Questions
Exam 23: Transition Metals Biological and Medical Applications119 Questions
Select questions type
Which of the following statements regarding the energy of a particle is NOT correct?
(Multiple Choice)
4.9/5  (35)
(35)
Work function values are often given in electron volts (1 eV = 1.602 * 10-19 J).You create an oxide material that has a work function of 6.10 eV.How many electrons would be emitted from the material per second when it is irradiated by the 172 nm light of a 55.0 W xenon excimer lamp, and what is their kinetic energy? Assume all of the incident photons cause electron emission.
(Multiple Choice)
4.9/5  (35)
(35)
How many electrons can be in the n = 4 shell of an atom? Include all orbitals that have (n = 4.)
(Short Answer)
4.9/5  (28)
(28)
The energy change for an electronic transition in a one-electron atom or ion H, He+, Li2+, etc.) from n initial to n final Is given by where Z is the atomic number.How much energy is required to ionize a ground-state Li2+ion (nfinal = )?
(Multiple Choice)
4.9/5  (28)
(28)
Which arrangement is correct for increasing atomic radius?
(Multiple Choice)
4.7/5  (33)
(33)
The work function of sodium is = 2.90 *10-19 J.What is the maximum wavelength that can cause the ejection of photoelectrons from a sodium surface?
(Multiple Choice)
4.9/5  (37)
(37)
The mathematical description of an electron as a wave was developed by _______.
(Multiple Choice)
4.7/5  (35)
(35)
What is the approximate total uncertainty in the velocity of an electron (9.11 *10-31 kg) confined within a gold atom whose diameter is about 270 pm? The uncertainty in the position of the electron is 5.0% of the atom's diameter.
(Multiple Choice)
4.8/5  (46)
(46)
What is the speed of an average argon atom that has a de Broglie wavelength of 5.2 pm?
(1 amu = 1.66054 *10-27 kg.)
(Multiple Choice)
4.7/5  (40)
(40)
Radiation with a wavelength of _______ nm is produced by the n = 4 to n = 2 transition in the hydrogen atom; this transition occurs in the _______ region of the electromagnetic spectrum.
(Short Answer)
4.9/5  (39)
(39)
Which of the transitions in the hydrogen atom energy-level diagram shown here is not possible? 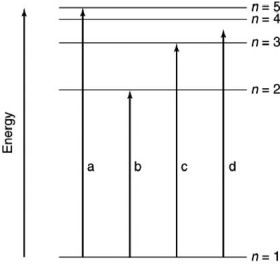
(Multiple Choice)
4.8/5  (29)
(29)
What is the difference in energy between the two electronic levels in hydrogen that are responsible for the emission line observed at 1282 nm, and to which series does this line belong?
(Multiple Choice)
4.7/5  (38)
(38)
What is the de Broglie wavelength of a fast neutron (1.6749 * 10-27 kg) traveling at 14,000 km/s?
(Multiple Choice)
4.7/5  (35)
(35)
Suppose a laser emits light in the visible region of the spectrum at 4.62 * 1014 s-1.What color is the light?
(Multiple Choice)
4.9/5  (33)
(33)
What is the uncertainty in the position of a helium atom traveling at 1360 m/s, approximately its speed at room temperature? The atom's velocity is known to about 1%.
(1 amu = 1.66054 * 10-27 kg.)
(Essay)
4.9/5  (42)
(42)
Which of the following statements regarding degenerate orbitals is correct?
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following atoms or ions has a single unpaired electron?
(Multiple Choice)
4.8/5  (40)
(40)
How much energy would be required to excite one mole of hydrogen atoms from their ground state to n2 = 10?
(Multiple Choice)
4.8/5  (38)
(38)
How many times longer is the wavelength of electromagnetic radiation used in a microwave oven at 2.45 GHz than the copper K- 1 x-ray radiation used in x-ray diffraction studies with an energy of 1.29 *10-15 J?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 101 - 120 of 175
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)