Exam 3: Atomic Structure Explaining the Properties of Elements
Exam 1: Matter and Energy an Atomic Perspective138 Questions
Exam 2: Atoms, Ions, and Molecules the Building Blocks of Matter143 Questions
Exam 3: Atomic Structure Explaining the Properties of Elements175 Questions
Exam 4: Chemical Bonding Understanding Climate Change182 Questions
Exam 5: Bonding Theories Explaining Molecular Geometry141 Questions
Exam 6: Intermolecular Forces Attractions Between Particles87 Questions
Exam 7: Stoichiometry Mass Relationships and Chemical Reactions140 Questions
Exam 8: Aqueous Solutions Chemistry of the Hydrosphere180 Questions
Exam 9: Thermochemistry Energy Changes in Chemical Reactions215 Questions
Exam 10: Properties of Gases the Air We Breathe164 Questions
Exam 11: Properties of Solutions Their Concentrations and Colligative Properties130 Questions
Exam 12: Thermodynamics Why Chemical Reactions Happen130 Questions
Exam 13: Chemical Kinetics Clearing the Air172 Questions
Exam 14: Chemical Equilibrium Equal but Opposite Reaction Rates119 Questions
Exam 15: Acid-Base Equilibria Proton Transfer in Biological Systems123 Questions
Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans114 Questions
Exam 17: Electrochemistry the Quest for Clean Energy135 Questions
Exam 18: The Solid State a Particulate View170 Questions
Exam 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials145 Questions
Exam 20: Biochemistry the Compounds of Life153 Questions
Exam 21: Nuclear Chemistry the Risks and Benefits168 Questions
Exam 22: The Main Group Elements Life and the Periodic Table116 Questions
Exam 23: Transition Metals Biological and Medical Applications119 Questions
Select questions type
Based on the following data and graph, which metal will emit the highest energy photoelectron when a 250 nm photon is incident on the metal? Work functions are in J * 10-19. 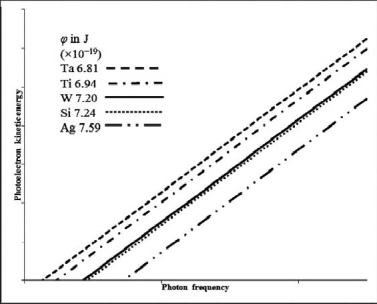
(Multiple Choice)
4.8/5  (38)
(38)
Place these types of radiation in order of increasing energy per photon.
I.yellow light from a street lamp
II.x-rays from a dental x-ray
III.microwaves from your cell phone
IV.radio waves from your favorite FM station
(Short Answer)
4.9/5  (45)
(45)
Which of the following statements about the aufbau principle is NOT true?
(Multiple Choice)
4.8/5  (40)
(40)
Light intensity (I) can be described as the power emitted by a light source (P) over a given area in meters squared).Suppose a 100 W mercury vapor lamp emits 365.4 nm photons equally in all directions.At a distance of 1.450 m, how many photons hit per square meter per second?
(1 W = 1 J/s; I = P/Area = P/4 d 2 , where d is the distance.)
(Multiple Choice)
4.8/5  (34)
(34)
De Broglie proposed that = h/mv.Define the symbols in this equation and describe the significance of de Broglie's proposal.
(Essay)
4.9/5  (36)
(36)
Which of the following is the correct electron configuration for copper?
(Multiple Choice)
4.8/5  (43)
(43)
Cesium atomic clocks are based on the emission of microwave radiation at 9,192,631,770 Hz by cesium-133.The wavelength corresponding to this frequency is closest in value to which of the following?
(Multiple Choice)
4.8/5  (46)
(46)
Electron affinity is the energy change that occurs _______.
(Multiple Choice)
4.9/5  (36)
(36)
Indicate which one of the following sources produces the highest energy photons.
(Multiple Choice)
4.8/5  (35)
(35)
The size of a typical atomic orbital is on the order of _______.
(Multiple Choice)
4.9/5  (45)
(45)
Identify which element, potassium or bromine, has the larger electron affinity and explain why.
(Essay)
4.9/5  (42)
(42)
In comparing the Fraunhofer lines with light emitted by elements in a flame, Bunsen and Kirchhoff demonstrated that _______.
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following types of electromagnetic radiation has the highest frequency?
(Multiple Choice)
4.9/5  (36)
(36)
Arrange the following elements in order of increasing largest negative) electron affinity: K, Ge, Kr, S, Si.
(Short Answer)
4.8/5  (42)
(42)
You synthesize an atom that appears to fit under francium in the periodic table.Which of the following would you predict to be the correct condensed electron configuration for this atom?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following elements would you expect to have the greatest first ionization energy?
(Multiple Choice)
4.9/5  (41)
(41)
The energy change for an electronic transition in a one-electron atom or ion H, He+, Li2+, etc.) from n initial to n final Is given by ,
where Z is the atomic number.
Which one of the following species will have the longest wavelength emission line for the transition between the ninitial = 2 and nfinal = 1 levels?
(Multiple Choice)
4.8/5  (39)
(39)
Which statement below about atoms containing more than one electron is FALSE?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 81 - 100 of 175
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)