Exam 3: Atomic Structure Explaining the Properties of Elements
Exam 1: Matter and Energy an Atomic Perspective138 Questions
Exam 2: Atoms, Ions, and Molecules the Building Blocks of Matter143 Questions
Exam 3: Atomic Structure Explaining the Properties of Elements175 Questions
Exam 4: Chemical Bonding Understanding Climate Change182 Questions
Exam 5: Bonding Theories Explaining Molecular Geometry141 Questions
Exam 6: Intermolecular Forces Attractions Between Particles87 Questions
Exam 7: Stoichiometry Mass Relationships and Chemical Reactions140 Questions
Exam 8: Aqueous Solutions Chemistry of the Hydrosphere180 Questions
Exam 9: Thermochemistry Energy Changes in Chemical Reactions215 Questions
Exam 10: Properties of Gases the Air We Breathe164 Questions
Exam 11: Properties of Solutions Their Concentrations and Colligative Properties130 Questions
Exam 12: Thermodynamics Why Chemical Reactions Happen130 Questions
Exam 13: Chemical Kinetics Clearing the Air172 Questions
Exam 14: Chemical Equilibrium Equal but Opposite Reaction Rates119 Questions
Exam 15: Acid-Base Equilibria Proton Transfer in Biological Systems123 Questions
Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans114 Questions
Exam 17: Electrochemistry the Quest for Clean Energy135 Questions
Exam 18: The Solid State a Particulate View170 Questions
Exam 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials145 Questions
Exam 20: Biochemistry the Compounds of Life153 Questions
Exam 21: Nuclear Chemistry the Risks and Benefits168 Questions
Exam 22: The Main Group Elements Life and the Periodic Table116 Questions
Exam 23: Transition Metals Biological and Medical Applications119 Questions
Select questions type
Which of the following types of electromagnetic radiation has the longest wavelength?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following is a possible set of quantum numbers for a 4 orbital?
(Multiple Choice)
4.7/5  (32)
(32)
Give the names of the three quantum numbers n, , and ml) that identify the mathematical functions orbitals) that are solutions to Schrödinger's wave equation.Describe how the energy, size, shape, and orientation of the orbital varies with the relative values of these quantum numbers.
(Essay)
5.0/5  (32)
(32)
If light at 193 nm from an argon-fluoride laser is incident on a gold-metal surface, electrons with a kinetic energy of 1.80 *10-19 J are produced.What is the work function of gold in kilojoules per mole? What is the minimum wavelength of light in nanometers required to produce photoelectrons from gold (zero kinetic energy)?
(Short Answer)
4.8/5  (34)
(34)
The de Broglie wavelength of buckyballs buckminsterfullerene, C60) has been observed experimentally.If a C60 molecule (1.20 *10-24 kg) has a velocity of 220 m/s, what is its de Broglie wavelength?
(Multiple Choice)
4.9/5  (43)
(43)
What is the ground-state electron configuration of an Al3+ion?
(Multiple Choice)
4.8/5  (36)
(36)
De Broglie reasoned that for the electron in the hydrogen atom to behave as a stable circular wave, the circumference of the electron's orbit must be _______.
(Multiple Choice)
4.8/5  (33)
(33)
A certain shell is known to have a total of 16 orbitals.Which shell is it?
(Multiple Choice)
4.8/5  (40)
(40)
Write the condensed electron configuration of Si.How many core and how many valence electrons does a silicon atom contain?
(Short Answer)
4.8/5  (40)
(40)
Which arrangement is in the correct order of decreasing radii?
(Multiple Choice)
4.8/5  (40)
(40)
Based on the following data and graph, indicate which metal requires the shortest wavelength photons to eject photoelectrons.Work functions are in J *10-19. 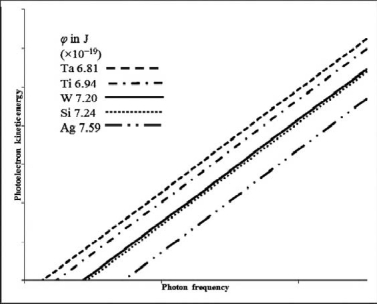
(Multiple Choice)
4.9/5  (44)
(44)
Showing 161 - 175 of 175
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)