Exam 11: Properties of Solutions Their Concentrations and Colligative Properties
Exam 1: Matter and Energy an Atomic Perspective138 Questions
Exam 2: Atoms, Ions, and Molecules the Building Blocks of Matter143 Questions
Exam 3: Atomic Structure Explaining the Properties of Elements175 Questions
Exam 4: Chemical Bonding Understanding Climate Change182 Questions
Exam 5: Bonding Theories Explaining Molecular Geometry141 Questions
Exam 6: Intermolecular Forces Attractions Between Particles87 Questions
Exam 7: Stoichiometry Mass Relationships and Chemical Reactions140 Questions
Exam 8: Aqueous Solutions Chemistry of the Hydrosphere180 Questions
Exam 9: Thermochemistry Energy Changes in Chemical Reactions215 Questions
Exam 10: Properties of Gases the Air We Breathe164 Questions
Exam 11: Properties of Solutions Their Concentrations and Colligative Properties130 Questions
Exam 12: Thermodynamics Why Chemical Reactions Happen130 Questions
Exam 13: Chemical Kinetics Clearing the Air172 Questions
Exam 14: Chemical Equilibrium Equal but Opposite Reaction Rates119 Questions
Exam 15: Acid-Base Equilibria Proton Transfer in Biological Systems123 Questions
Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans114 Questions
Exam 17: Electrochemistry the Quest for Clean Energy135 Questions
Exam 18: The Solid State a Particulate View170 Questions
Exam 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials145 Questions
Exam 20: Biochemistry the Compounds of Life153 Questions
Exam 21: Nuclear Chemistry the Risks and Benefits168 Questions
Exam 22: The Main Group Elements Life and the Periodic Table116 Questions
Exam 23: Transition Metals Biological and Medical Applications119 Questions
Select questions type
Determine the molal concentration of a table sugar (C12H22O11, 342.3 g/mol) solution in water that has a freezing point of -2.10 C.(Kf =1.86 C/m for water)
(Multiple Choice)
4.9/5  (28)
(28)
The smell of fresh-cut pine is due in part to a cyclic alkene called pinene.A graph of the natural logarithm of the vapor pressure of pinene vs.1/temperature produces a straight line with a slope of -4936.37 K.What is the enthalpy of vaporization of pinene?
(Multiple Choice)
4.8/5  (43)
(43)
Gasoline is primarily a mixture of hydrocarbons and is sold with an octane rating that is based on a comparison with the properties of isooctane (C8H18), which has an enthalpy of vaporization of 35.8 kJ/mol and a normal boiling point of 98.2 C.Determine the vapor pressure of isooctane on a very hot day when the temperature is 38.0 C.
(Multiple Choice)
4.8/5  (40)
(40)
What is the freezing point of an aqueous solution prepared by dissolving 113 g of sodium phosphate (Na3PO4, 163.94 g/mol) in 2.50 kg of water? Use the ideal van 't Hoff factor.(Kf (water) =1.86 C/m)
(Short Answer)
4.9/5  (37)
(37)
What is the boiling point of a solution prepared by dissolving 10.00 g of naphthalene (C10H8, 128.17 g/mol) in 200.0 g of benzene (C6H6, 78.12 g/mol)? The normal boiling point of benzene is 80.1 C and Kb =2.53 C/m.
(Multiple Choice)
4.8/5  (25)
(25)
Which of the following statements regarding solubility of gases in liquids is NOT correct?
(Multiple Choice)
4.8/5  (29)
(29)
Does the temperature of the vapors in a still head increase or decrease as a fractional distillation progresses? Explain.
(Essay)
4.7/5  (32)
(32)
Water contains about 42 mg of oxygen per liter at 20 C and 1 atm.What is the Henry's law constant for oxygen dissolving in water?
(Multiple Choice)
4.8/5  (37)
(37)
A 275 mg sample of a nonelectrolyte compound was dissolved in water to produce 10.0 mL of a solution at 25 C.The osmotic pressure of this solution was measured and found to be 3.67 atm.What is the molar mass of this compound?
(Multiple Choice)
4.8/5  (36)
(36)
The arrow in the diagram below indicates the direction of solvent flow through a membrane in osmosis.Which solution, A or B, is more concentrated? Explain your reasoning. 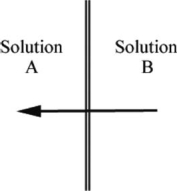
(Essay)
4.8/5  (41)
(41)
Calculate the molality of a solution containing 0.575 mol of ethanol (CH3CH2OH) and 395 g of water.
(Multiple Choice)
4.7/5  (39)
(39)
A solution contains 6.50 mol of water, 0.300 mol of sucrose, and 0.200 mol of glucose.The solutes are nonvolatile.What is the vapor pressure of the solution at 35 C given that the vapor pressure of water is 42.2 torr?
(Multiple Choice)
4.7/5  (37)
(37)
Calculate the minimum pressure that must be applied to achieve reverse osmosis of 0.320 M KBr at 25.0 C.
(Multiple Choice)
4.8/5  (41)
(41)
A solution contains two isomers, n-propyl alcohol and isopropyl alcohol, at 25 C.The total vapor pressure is 38.6 torr.What are the mole fractions of each alcohol in the liquid and in the vapor phase? The vapor pressures are 21.0 torr for n-propyl alcohol and 45.2 torr for isopropyl alcohol.
(Essay)
4.7/5  (47)
(47)
The aroma from almonds and cherries is due in part to a compound called benzaldehyde.A graph of the natural logarithm of the vapor pressure of benzaldehyde vs.1/temperature produces a straight line with a slope of -5870.99 K.What is the enthalpy of vaporization of benzaldehyde?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following solutions is isotonic with the solution inside a red blood cell, which has an osmotic pressure of 7.89 atm at 37.0 C?
(Multiple Choice)
4.8/5  (28)
(28)
Which solution will have the lowest osmotic pressure when measured against pure water?
(Multiple Choice)
5.0/5  (36)
(36)
How many grams of potassium nitrate (KNO3) must be added to 450.0 g of water to produce a 1.30 m solution?
(Multiple Choice)
4.8/5  (33)
(33)
Indicate which aqueous solution has the slowest evaporation rate.
(Multiple Choice)
4.9/5  (44)
(44)
Showing 21 - 40 of 130
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)