Exam 11: Properties of Solutions Their Concentrations and Colligative Properties
Exam 1: Matter and Energy an Atomic Perspective138 Questions
Exam 2: Atoms, Ions, and Molecules the Building Blocks of Matter143 Questions
Exam 3: Atomic Structure Explaining the Properties of Elements175 Questions
Exam 4: Chemical Bonding Understanding Climate Change182 Questions
Exam 5: Bonding Theories Explaining Molecular Geometry141 Questions
Exam 6: Intermolecular Forces Attractions Between Particles87 Questions
Exam 7: Stoichiometry Mass Relationships and Chemical Reactions140 Questions
Exam 8: Aqueous Solutions Chemistry of the Hydrosphere180 Questions
Exam 9: Thermochemistry Energy Changes in Chemical Reactions215 Questions
Exam 10: Properties of Gases the Air We Breathe164 Questions
Exam 11: Properties of Solutions Their Concentrations and Colligative Properties130 Questions
Exam 12: Thermodynamics Why Chemical Reactions Happen130 Questions
Exam 13: Chemical Kinetics Clearing the Air172 Questions
Exam 14: Chemical Equilibrium Equal but Opposite Reaction Rates119 Questions
Exam 15: Acid-Base Equilibria Proton Transfer in Biological Systems123 Questions
Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans114 Questions
Exam 17: Electrochemistry the Quest for Clean Energy135 Questions
Exam 18: The Solid State a Particulate View170 Questions
Exam 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials145 Questions
Exam 20: Biochemistry the Compounds of Life153 Questions
Exam 21: Nuclear Chemistry the Risks and Benefits168 Questions
Exam 22: The Main Group Elements Life and the Periodic Table116 Questions
Exam 23: Transition Metals Biological and Medical Applications119 Questions
Select questions type
The vapor pressure of an aqueous solution is found to be 24.9 mmHg at 25 C.What is the mole fraction of solute in this solution? The vapor pressure of water is 25.756 mmHg at 25 C.
(Multiple Choice)
4.8/5  (37)
(37)
Define the term "colligative" in relation to the properties of a solution and list four colligative properties.
(Essay)
4.9/5  (41)
(41)
Which solution, if either, would create the higher osmotic pressure compared to pure water): one prepared from 1.0 g of NaCl in 10 mL of water or one prepared from 1.0 g of CsBr in 10 mL of water?
(Multiple Choice)
4.9/5  (43)
(43)
Henry's law constant for oxygen dissolving in blood is 3.74 *10-2 mol/L . atm at body temperature, 37 C.Calculate the molar concentration of oxygen in blood for a free diver where the air pressure is 3.0 atm.The mole fraction of oxygen in air is 0.209.
C.Calculate the molar concentration of oxygen in blood for a free diver where the air pressure is 3.0 atm.The mole fraction of oxygen in air is 0.209.
(Short Answer)
4.7/5  (42)
(42)
A solution is prepared by adding 0.300 mol of glucose, which is not volatile, to 4.50 mol of water.What is the vapor pressure of this solution at 25 C given that the vapor pressure of pure water is 23.8 torr?
(Multiple Choice)
4.9/5  (38)
(38)
Indicate which aqueous solution has the lowest vapor pressure.
(Multiple Choice)
4.9/5  (39)
(39)
The normal freezing point of benzene (C6H6, 78.12 g/mol) is 5.49 C, and its Kf value is 5.12 C/m.To create a solution with a freezing point of 0.00 C, how many grams of naphthalene (C10H8, 128.2 g/mol) would need to be dissolved in 640.0 g of benzene?
(Multiple Choice)
4.8/5  (44)
(44)
You wish to de-ice your driveway, so you consider buying 10 lb of NaCl, 10 lb of CaCl2, or 10 lb of MgCl2.Which of these would de-ice your driveway most effectively? Assume that ideal dissociation of the salts occurs and that colligative properties are the only consideration.
(Multiple Choice)
4.9/5  (43)
(43)
A 15.0 mg sample of a protein was dissolved in water to produce 5.00 mL of solution at 25.1 C.The osmotic pressure of this solution was measured and found to be 6.50 torr.What is the molar mass of this protein?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following statements regarding solutions and the van 't Hoff factor is NOT correct?
(Multiple Choice)
4.9/5  (47)
(47)
What would be the freezing point of a 1.50 m solution of NaNO3 in water? (Kf = 1.86 C)
(Multiple Choice)
4.8/5  (38)
(38)
A 0.512 g sample of an unknown nondissociating solute was dissolved in 25.0 g of camphor (Kf = 39.7 C/m), decreasing the freezing point of camphor by 3.07 C.What is the molar mass of the solute?
(Multiple Choice)
4.8/5  (44)
(44)
How does the presence of solutes raise the boiling point of a solvent?
(Essay)
4.9/5  (38)
(38)
Lanterns and stoves that use n-pentane as a fuel are often difficult to light on a cold day because the fuel has a low vapor pressure at low temperatures.Determine the vapor pressure of n-pentane on a night when the temperature is 0.0 C.The enthalpy of vaporization of n-pentane is 27.6 kJ/mol, and its boiling point is 36.0 C.
(Multiple Choice)
4.9/5  (33)
(33)
Identify the following statement as true or false and choose the correct explanation: "For solutions with the same molarity at the same temperature, the pressure needed for reverse osmosis of a sodium chloride solution will always be less than the pressure needed for a calcium chloride solution."
(Multiple Choice)
4.8/5  (41)
(41)
A solution is prepared by mixing 45.68 g of carbon disulfide (CS2, 76.13 g/mol) with 16.42 g of acetonitrile (CH3CN, 41.06 g/mol).What is the mole fraction of CS2 in the vapor phase at 25 C? 
(Multiple Choice)
4.8/5  (30)
(30)
Which statement regarding the boiling of a mixture of liquids A and B is NOT correct? 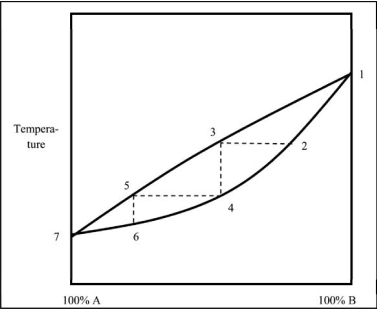
(Multiple Choice)
4.9/5  (36)
(36)
In the process of dialysis, a special semipermeable membrane allows both small molecules and water to pass through, but not large protein molecules.These membranes are used to separate these small molecules and ions from much larger proteins.If a mixture of proteins and small molecules were separated from pure water by a dialysis membrane as shown in the figure, which way would the molecules flow? 
(Multiple Choice)
4.8/5  (39)
(39)
What is the vapor pressure of an aqueous solution that has a solute mole fraction of =0.200? The vapor pressure of water is 25.756 mmHg at 25 C.
(Multiple Choice)
4.9/5  (43)
(43)
The normal boiling point of ammonia is -33.34 C, and its enthalpy of vaporization is 23.35 kJ/mol.What pressure would have to be applied for ammonia to boil at 25.00 C?
(Multiple Choice)
4.9/5  (36)
(36)
Showing 101 - 120 of 130
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)