Exam 2: Water the Solvent for Biochemical Reactions
Exam 1: Biochemistry and the Organization of Cells76 Questions
Exam 2: Water the Solvent for Biochemical Reactions90 Questions
Exam 3: Amino Acids and Peptides80 Questions
Exam 4: The Three Dimensional Structure of Proteins87 Questions
Exam 5: Protein Purification and Characterization Techniques72 Questions
Exam 6: The Behavior of Proteins Enzymes88 Questions
Exam 7: The Behavior of Proteins Enzymes Mechanisms and Control86 Questions
Exam 8: Lipids and Proteins Are Associated in Biological Membranes95 Questions
Exam 9: Nucleic Acids How Structure Conveys Information71 Questions
Exam 10: Biosynthesis of Nucleic Acids Replication91 Questions
Exam 11: Transcription of the Genetic Code the Biosynthesis of Rna103 Questions
Exam 12: Protein Synthesis Translation of the Genetic Message90 Questions
Exam 13: Nucleic Acid Biotechnology Techniques99 Questions
Exam 14: Viruses Cancer and Immunology47 Questions
Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism65 Questions
Exam 16: Carbohydrates97 Questions
Exam 17: Glycolysis72 Questions
Exam 18: Storage Mechanisms and Control in Carbohydrate Metabolism87 Questions
Exam 19: The Citric Acid Cycle85 Questions
Exam 20: Electron Transport and Oxidative Phosphorylation71 Questions
Exam 21: Lipid Metabolism100 Questions
Exam 22: Photosynthesis79 Questions
Exam 23: The Metabolism of Nitrogen83 Questions
Exam 24: Integration of Metabolism Cellular Signaling73 Questions
Select questions type
The tendency for an atom to attract electrons to itself in a chemical bond is called
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following statements is true of dipole-induced dipole interactions?
(Multiple Choice)
4.8/5  (40)
(40)
Hydrogen bonds explain which of the following properties of water?
(Multiple Choice)
4.9/5  (35)
(35)
For an acid that undergoes this reaction:
HA ↔ H+ + A −
Ka =
(Multiple Choice)
4.9/5  (29)
(29)
When a carboxylate side-chain of one amino acid in a protein is in close proximity to a charged amino group of another amino acid, we call the resulting interaction a(n)
(Multiple Choice)
4.8/5  (30)
(30)
Consider a reaction that produces a significant amount of hydrogen ion and is to be carried out a pH 7. Only two acids are available for making the buffer solution. The pKa values for acids A and B are 6.3 and 7.3, respectively. Which acid would serve as the optimum buffer for this reaction? Or would carrying out the reaction in water simply serve as well?
(Multiple Choice)
4.8/5  (34)
(34)
Ionic compounds and polar covalent compounds tend to dissolve in water because of
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following compounds is most likely to form a micelle?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following properties of water are related to its ability to form hydrogen bonds?
(Multiple Choice)
4.7/5  (42)
(42)
An HCl solution has a pH = 3. If you dilute 10 mL of the solution to 1000mL, the final pH will be:
(Multiple Choice)
4.9/5  (27)
(27)
The dissociation constant for an acid with a pKa value of 6.0 is
(Multiple Choice)
4.8/5  (39)
(39)
What is the pH of an acetic acid solution where the concentration of acetic acid is 2 mM and the concentration of sodium acetate is 20 mM. The pKa of acetic acid is 4.76.
(Multiple Choice)
4.9/5  (37)
(37)
Exhibit 2A The structure of ATP with various groups labeled. Group III is the entire phosphate group. 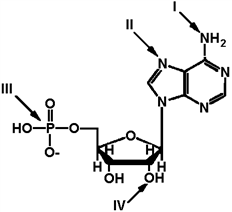 Refer to Exhibit 2A. Which of the functional groups is the most electrophilic?
Refer to Exhibit 2A. Which of the functional groups is the most electrophilic?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 61 - 80 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)