Exam 2: Water the Solvent for Biochemical Reactions
Exam 1: Biochemistry and the Organization of Cells76 Questions
Exam 2: Water the Solvent for Biochemical Reactions90 Questions
Exam 3: Amino Acids and Peptides80 Questions
Exam 4: The Three Dimensional Structure of Proteins87 Questions
Exam 5: Protein Purification and Characterization Techniques72 Questions
Exam 6: The Behavior of Proteins Enzymes88 Questions
Exam 7: The Behavior of Proteins Enzymes Mechanisms and Control86 Questions
Exam 8: Lipids and Proteins Are Associated in Biological Membranes95 Questions
Exam 9: Nucleic Acids How Structure Conveys Information71 Questions
Exam 10: Biosynthesis of Nucleic Acids Replication91 Questions
Exam 11: Transcription of the Genetic Code the Biosynthesis of Rna103 Questions
Exam 12: Protein Synthesis Translation of the Genetic Message90 Questions
Exam 13: Nucleic Acid Biotechnology Techniques99 Questions
Exam 14: Viruses Cancer and Immunology47 Questions
Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism65 Questions
Exam 16: Carbohydrates97 Questions
Exam 17: Glycolysis72 Questions
Exam 18: Storage Mechanisms and Control in Carbohydrate Metabolism87 Questions
Exam 19: The Citric Acid Cycle85 Questions
Exam 20: Electron Transport and Oxidative Phosphorylation71 Questions
Exam 21: Lipid Metabolism100 Questions
Exam 22: Photosynthesis79 Questions
Exam 23: The Metabolism of Nitrogen83 Questions
Exam 24: Integration of Metabolism Cellular Signaling73 Questions
Select questions type
Which of the following molecules will not form hydrogen bonds?
(Multiple Choice)
4.8/5  (40)
(40)
An ammonia buffer contains NH3:NH4+ in a ratio of 0.4 moles:0.6 moles (pK = 9.75). What will be the pH if you add 0.01 moles of HCl to this buffer?
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following molecules has polar bonds but is itself not polar?
(Multiple Choice)
4.8/5  (32)
(32)
How do hydrogen bonds tend to affect the melting and boiling points of substances?
(Multiple Choice)
4.7/5  (36)
(36)
A buffer solution at pH 10 has a ratio of [HA] \[A − ] of 10. What is the pKa of the acid?
(Multiple Choice)
4.8/5  (38)
(38)
Exhibit 2A The structure of ATP with various groups labeled. Group III is the entire phosphate group. 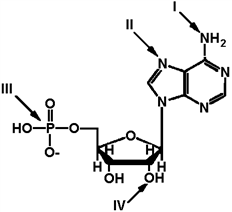 Refer to Exhibit 2A. Which of the functional groups cannot function as a hydrogen donor to water?
Refer to Exhibit 2A. Which of the functional groups cannot function as a hydrogen donor to water?
(Multiple Choice)
4.8/5  (36)
(36)
The inflection point of the titration curve for a weak monoprotic acid is equal to its pKa
(True/False)
4.9/5  (39)
(39)
Exhibit 2B Contains information on the pK's of some common buffers. 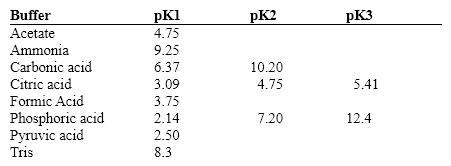 Refer to Exhibit 2B. A carbonate buffer would work well at this pH:
Refer to Exhibit 2B. A carbonate buffer would work well at this pH:
(Multiple Choice)
4.8/5  (40)
(40)
Exhibit 2B Contains information on the pK's of some common buffers. 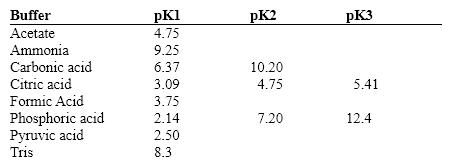 Refer to Exhibit 2B. A phosphate buffer would work well at this pH:
Refer to Exhibit 2B. A phosphate buffer would work well at this pH:
(Multiple Choice)
4.7/5  (32)
(32)
If atoms with greatly differing electronegativities form a bond, that bond will be
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following elements has the highest electronegativity?
(Multiple Choice)
4.8/5  (37)
(37)
Using the Henderson-Hasselbalch equation, calculate the pH of an ammonia buffer when the NH3:NH4+ ratio is 0.4 moles:0.6 moles. (pK = 9.75)
(Multiple Choice)
4.9/5  (32)
(32)
Calculate the final pH of a solution made by the addition of 10 mL of a 0.5 M NaOH solution to 500 mL of a 0.4 M HA originally at pH = 5.0 (pKa = 5.0) Neglect the volume change.
(Multiple Choice)
4.9/5  (33)
(33)
Molecules which contain both hydrophilic and hydrophobic regions are:
(Multiple Choice)
4.8/5  (40)
(40)
If the pH of 1 liter of a 1.0 M carbonate buffer is 7.0, what is actual number of moles of H2CO3 and HCO3 − ? (pK = 6.37) 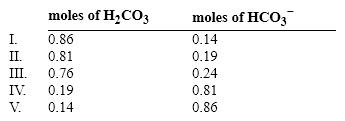
(Multiple Choice)
4.9/5  (39)
(39)
Showing 41 - 60 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)