Exam 7: Electronic Structure
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
Scientists of the 19th Century found that the equation, 1/ l = RH(1/n21 - 1/n22) (where RH = 1.097×107 m - 1, and n1 and n2 are whole numbers) predicts the wavelength of the lines in the emission spectrum of the H atom. This equation is properly referred to as:
(Multiple Choice)
4.8/5  (38)
(38)
Exhibit 7-1 Consider the shapes listed below to answer the following question(s):
I.  II.
II.  III.
III. 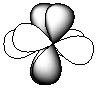 IV.
IV. 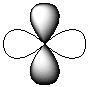 V.
V. 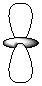 -Refer to Exhibit 7-1. Which of the shapes would represent one of seven possible 4f atomic orbitals?
-Refer to Exhibit 7-1. Which of the shapes would represent one of seven possible 4f atomic orbitals?
(Multiple Choice)
4.9/5  (35)
(35)
What is the correct electronic configuration for Arsenic (Z = 33) taking into account the proper order for filling subshells?
(Multiple Choice)
4.8/5  (40)
(40)
What is the energy of one photon of radiation with a wavelength of 325 nm?
(Multiple Choice)
4.9/5  (45)
(45)
Which neutral element has an electronic configuration of 1s22s22p63s23p64s23d6?
(Multiple Choice)
4.8/5  (35)
(35)
What quantum number(s) provide(s) information with respect to the shape of an atomic orbital?
(Multiple Choice)
4.9/5  (29)
(29)
What quantum number(s) is(are) required to completely describe an orbital , the region in space in which an electron has a high probability of being found?
I. Principal quantum number
II. Angular Momentum quantum number
III. Magnetic quantum number
(Multiple Choice)
4.7/5  (47)
(47)
Which of the following atoms is paramagnetic in the ground state?
(Multiple Choice)
4.8/5  (33)
(33)
Which electronic configuration(s) listed below is(are) correct for the elements provided?
I. Nitrogen 1s22s22p3
II. Tin [Kr]5s24d105p2
III. Bromine 1s22s22p63s23p63d104s24p7
(Multiple Choice)
4.9/5  (33)
(33)
What is the correct electronic configuration for Zirconium (Z = 40)?
(Multiple Choice)
4.9/5  (43)
(43)
What is the energy of a photon of light that has a frequency of 2.3×1014 s - 1 (h = 6.6×10 - 34 J × s)?
(Multiple Choice)
4.7/5  (36)
(36)
How many possible orientations are allowed when 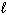 = 0?
(s-type sublevel; How many possible
= 0?
(s-type sublevel; How many possible 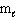 values are possible?)
values are possible?)
(Multiple Choice)
4.7/5  (44)
(44)
How many possible orientations are allowed when 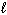 = 3?
(f-type sublevel; How many possible
= 3?
(f-type sublevel; How many possible 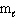 values are possible?)
values are possible?)
(Multiple Choice)
4.9/5  (46)
(46)
An argon ion laser emits light at 489 nm. What is the frequency of this radiation?
(Multiple Choice)
4.7/5  (43)
(43)
What is the ground state electron configuration of fluorine?
(Multiple Choice)
4.8/5  (34)
(34)
What quantum number(s) provide(s) information with respect to the spatial orientation of an atomic orbital?
(Multiple Choice)
4.8/5  (32)
(32)
Which orbital diagram for the ground state electronic configuration of a carbon atom is correct?
(Remember Hund's Rule)
(Multiple Choice)
4.9/5  (39)
(39)
Calculate the wavelength of light emitted when an electron in a He+ ion goes from the n = 5 shell to the n = 2 shell.
(Multiple Choice)
4.8/5  (31)
(31)
Showing 41 - 60 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)