Exam 7: Electronic Structure
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
Only one set of quantum numbers listed below is a correct set. Which one?
(n, l , m l , ms)
(Multiple Choice)
4.9/5  (28)
(28)
How many electrons can be placed in the subshell described by the quantum numbers n = 3, 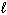 = 1?
= 1?
(Multiple Choice)
4.8/5  (31)
(31)
What is the wavelength of light with a frequency of 8.2×1013 s - 1?
(Multiple Choice)
4.9/5  (29)
(29)
The number of wave crests that pass a fixed point each second is known as the ____ of the wave.
(Multiple Choice)
4.8/5  (36)
(36)
The energy of the electron in the hydrogen atom depends on the quantum number(s)
(Multiple Choice)
4.8/5  (41)
(41)
How many possible orientations are allowed when 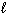 = 1?
(p-type sublevel; How many possible
= 1?
(p-type sublevel; How many possible 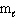 values are possible?)
values are possible?)
(Multiple Choice)
4.9/5  (40)
(40)
According to the Bohr model for a hydrogen atom, the lines on the hydrogen emission spectrum are related to what type of energy change?
(Multiple Choice)
4.8/5  (29)
(29)
Calculate the wavelength of light emitted when an electron in a hydrogen atom falls from the n = 4 orbit to the n = 1 orbit (Rh = 1.1×107 m - 1).
(Multiple Choice)
4.7/5  (33)
(33)
Given the three statements below, which answer is best?
I. Although the wavelength of light and the frequency of light can change, the energy remains constant.
II. An important consequence of quantum theory is that the energy of various electronic states for atoms are at fixed discrete levels.
III. The energy of an electronic state for a hydrogen atom is dependent only on the principal quantum number n.
(Multiple Choice)
4.9/5  (30)
(30)
Three sets of quantum numbers are listed below. Pick the best answer.
I. n = 3, 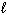 = 3,
= 3, 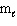 = 2
II. n = 4,
= 2
II. n = 4, 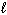 = 2,
= 2, 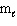 = 0
III. n = 1,
= 0
III. n = 1, 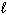 = 0,
= 0, 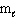 = 0
= 0
(Multiple Choice)
4.7/5  (36)
(36)
Given the three statements below, pick the best answer.
I. c = ln
II. E = h n
III. heating gaseous Li atoms yields white light
(Multiple Choice)
4.8/5  (33)
(33)
Which orbital diagram for the ground state electronic configuration of an oxygen atom is correct?
(Multiple Choice)
4.9/5  (35)
(35)
Which set(s) of three quantum numbers is(are) permissible?
I. n = 2, 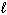 = 1, and
= 1, and 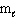 = 1
II. n = 4,
= 1
II. n = 4, 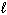 = 2, and
= 2, and 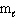 = - 2
III. n = 3,
= - 2
III. n = 3, 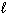 = 3, and
= 3, and 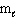 = 0
= 0
(Multiple Choice)
4.8/5  (33)
(33)
Exhibit 7-1 Consider the shapes listed below to answer the following question(s):
I.  II.
II.  III.
III. 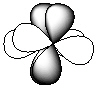 IV.
IV. 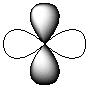 V.
V. 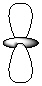 -Refer to Exhibit 7-1. Which of the shapes would represent one of three possible 2p atomic orbitals?
-Refer to Exhibit 7-1. Which of the shapes would represent one of three possible 2p atomic orbitals?
(Multiple Choice)
4.8/5  (36)
(36)
Which full set of four quantum numbers would be expected for the highest energy electron for a potassium atom?
(Multiple Choice)
4.9/5  (43)
(43)
From the restrictions on the three quantum numbers that define an orbital, which set of three quantum numbers listed below is forbidden?
(Multiple Choice)
4.9/5  (35)
(35)
What is the correct electronic configuration for the element Manganese (Z = 25)?
(Multiple Choice)
4.7/5  (33)
(33)
What is the order for filling the following atomic subshells?
(Lowest energy to highest energy) 4f, 5p, 5d, 6s
(Multiple Choice)
4.8/5  (33)
(33)
Showing 61 - 80 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)