Exam 7: Electronic Structure
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
The threshold frequency for the photoelectric effect of a particular metal is 3.0×1014 sec - 1. When this metal is illuminated by light with a frequency of 2.0×1014 sec - 1, which of the following is true?
(Multiple Choice)
4.8/5  (38)
(38)
How much energy does a single photon of an argon ion laser emit if the wavelength of this photon is 489 nm?
(Multiple Choice)
4.8/5  (42)
(42)
Calculate the frequency of light whose wavelength is 4.2×10 - 7 meters.
(Multiple Choice)
4.9/5  (29)
(29)
Three sets of quantum numbers are given below. Choose the best answer.
I. n = 3, 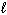 = 3,
= 3, 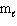 = 0, m s = 1 / 2
II. n = 2,
= 0, m s = 1 / 2
II. n = 2, 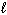 = 1,
= 1, 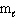 = 1, m s = 1 / 2
III. n = 4,
= 1, m s = 1 / 2
III. n = 4, 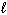 = 2,
= 2, 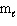 = 3, m s = - 1 / 2
= 3, m s = - 1 / 2
(Multiple Choice)
4.8/5  (38)
(38)
The energy of a photon of electromagnetic radiation with a wavelength of 1.0 nm is
(Multiple Choice)
4.9/5  (33)
(33)
Which orbital diagram for the ground state electronic configuration of a Nitrogen atom is correct?
(Multiple Choice)
4.9/5  (37)
(37)
How many electrons can be placed in the subshell described by the quantum numbers n = 2, 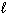 = 0?
= 0?
(Multiple Choice)
4.8/5  (42)
(42)
What is the maximum number of electrons that can occupy a 5d sublevel?
(Multiple Choice)
4.8/5  (37)
(37)
A subshell is described by assigning values to the quantum numbers
(Multiple Choice)
5.0/5  (46)
(46)
What is the correct electronic configuration for Bromine (Z = 35) taking into account the proper order for filling the subshells?
(Multiple Choice)
4.9/5  (33)
(33)
The possible values of the magnetic quantum number, 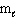 , of a 3d electron are:
, of a 3d electron are:
(Multiple Choice)
5.0/5  (45)
(45)
Which equation below properly relates energy (E) to wavelength ( l ) given h = Plank's constant, c = speed of light, and n = frequency?
(Multiple Choice)
4.8/5  (31)
(31)
The element with a ground state electron configuration of 1s22s22p6 is
(Multiple Choice)
4.8/5  (34)
(34)
The distance between two adjacent peaks of a wave is known as the ____ of the wave.
(Multiple Choice)
4.8/5  (29)
(29)
The possible values of the magnetic quantum number, 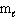 , of a 2p electron are:
, of a 2p electron are:
(Multiple Choice)
4.8/5  (29)
(29)
Calculate the frequency of light whose wavelength is 711 nm.
(Multiple Choice)
4.8/5  (28)
(28)
The energy of a photon of electromagnetic radiation with a frequency of 1.5×1014 Hz is
(Multiple Choice)
4.8/5  (36)
(36)
Showing 21 - 40 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)