Exam 15: Solutions of Acids and Bases
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
Measurements show that the [OH - ] of a solution is 6.02×10 - 6 M. The pH of this solution is:
(Multiple Choice)
4.8/5  (31)
(31)
Consider the following unfinished table of weak acids with their corresponding p K a values and K a values. Weak Acid p K a K a Benzoic acid 4.20 -- Lactic acid -- 1.4×10 - 4 Chlorous acid 1.96 -- Arrange these three acids in order of increasing acid strength .
(Multiple Choice)
4.8/5  (37)
(37)
Exhibit 15-5 Consider solving for the pH of a 0.300 M aqueous solution of KCN to answer the following question(s).
-Refer to Exhibit 15-5. What is the [OH - ] ion concentration in this 0.300 M KCN aqueous solution?
(Multiple Choice)
4.9/5  (38)
(38)
What species are formed in the hydrolysis reaction of KCN with water?
KCN + H₂ O 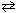 ??
??
(Multiple Choice)
4.9/5  (34)
(34)
Identify the Lewis acid in the following reaction. CO2 + H2O→H2CO3
(Multiple Choice)
4.8/5  (42)
(42)
Consider the changes in acid strengths in the series HClO, HClO2, HClO3, and HClO4.
(Multiple Choice)
4.8/5  (37)
(37)
What is the pH of 2.0 liters of aqueous solution that contains 9.0 grams of HCl?
(Multiple Choice)
4.7/5  (27)
(27)
Exhibit 15-2 The pH of a solution of a certain brand of Cola is 2.27. Use this information to solve the following problem(s).
Refer to Exhibit 15-2. What is the hydroxide ion concentration, [OH - ], of this solution?
(Multiple Choice)
5.0/5  (31)
(31)
A water soluble salt composed of the anion of a weak acid and the cation of a strong base is dissolved in pure water. The resulting solution:
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following substances would be the strongest acid?
(Multiple Choice)
4.8/5  (42)
(42)
Benzoic acid is weak with K a = 6.3×10 - 5. What fraction ionizes in a 0.050 M solution?
(Multiple Choice)
4.9/5  (45)
(45)
Exhibit 15-1 The pH of a certain brand of milk is 6.60. Use this information to answer the following question(s).
-Refer to Exhibit 15-1. What is the molar concentration of hydroxide ion , [OH - ], in this milk?
(Multiple Choice)
4.8/5  (40)
(40)
Exhibit 15-1 The pH of a certain brand of milk is 6.60. Use this information to answer the following question(s).
-Refer to Exhibit 15-1. What is the molar concentration of the hydronium ion , [H3O+], in this milk?
(Multiple Choice)
4.8/5  (42)
(42)
Exhibit 15-5 Consider solving for the pH of a 0.300 M aqueous solution of KCN to answer the following question(s).
-Refer to Exhibit 15-5. What is the pH of this 0.300 M KCN aqueous solution?
(Multiple Choice)
4.9/5  (48)
(48)
Milk of magnesia is essentially a saturated solution of Mg(OH)2 ( K sp = 8.9×10 - 12). What is the pH of a saturated Mg(OH)2 solution?
(Multiple Choice)
4.9/5  (42)
(42)
Showing 41 - 60 of 179
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)