Exam 15: Solutions of Acids and Bases
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
Which of the following salts is(are) considered basic when dissolved in water?
I. NaNO3
II. K3PO4
III. NH4Cl
(Multiple Choice)
4.8/5  (34)
(34)
A famous brand of sweet and sour barbecue sauce (consisting of tomato paste, water, vinegar, sugar, and secret spices) has a measured pH of 2.6. What is the hydrogen ion concentration in the sauce?
(Multiple Choice)
4.9/5  (31)
(31)
Rank the following three acids in terms of acid strength given the following information:
Acid K a p K a HF 6.9×10 - 4 -- CH3COOH -- 4.74 HNO2 4.5×10 - 4
(Multiple Choice)
4.9/5  (39)
(39)
Exhibit 15-4 Consider dissolving NaF in water as shown in the equation below to answer the following question(s):
NaF (s) + H₂ O ( 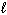 )
) 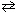 HF (aq) + NaOH (aq)
-Refer to Exhibit 15-4. What is the pH of this 0.100 M NaF solution?
HF (aq) + NaOH (aq)
-Refer to Exhibit 15-4. What is the pH of this 0.100 M NaF solution?
(Multiple Choice)
4.9/5  (30)
(30)
What is the pH of an aqueous 0.50 M KCN solution?
K a(HCN) = 6.2×10 - 10
(Multiple Choice)
4.8/5  (39)
(39)
The pH of a 1.00 liter solution of the weak base nicotine ( K b = 1.05×10 - 6) is 10.20.
How many moles of nicotine were dissolved to form this solution?
(Multiple Choice)
4.8/5  (32)
(32)
The pH of a vinegar solution is 2.32. What is the hydronium ion concentration, [H3O+], for this solution?
(Multiple Choice)
4.8/5  (39)
(39)
Regarding acid strength, which of the following is not correctly assigned?
(Multiple Choice)
4.8/5  (42)
(42)
Consider the salt pyridinium acetate, [(C5H5NH+)(C2H3O21 - )],
That is derived from a combination of a weak acid , HC2H3O2, and a weak base , C5H5N,
As shown below.
C5H5N + HC2H3O2→[(C5H5NH+)(C2H3O21 - )] K b(C5H5N) = 1.7×10 - 9 and K a(HC2H3O2) = 1.8×10 - 5 Which statement listed below is true?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following substances would behave as a Lewis acid when combined with a substance such as H2O?
I. BeF2
II. Al3+
III. H+
(Multiple Choice)
4.8/5  (32)
(32)
What species are formed in the hydrolysis of KF with water ?
KF + H₂ O 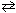 ??
??
(Multiple Choice)
4.8/5  (35)
(35)
For the reaction CN - (aq) + H₂ O ( 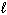 )
)  HCN (aq) + OH - (aq), one of the acid-base conjugate pairs is:
HCN (aq) + OH - (aq), one of the acid-base conjugate pairs is:
(Multiple Choice)
5.0/5  (38)
(38)
What is the pH of a 0.10 M HCN solution?
K a(HCN) = 4.9×10 - 10
(Multiple Choice)
4.9/5  (33)
(33)
After completing the following unfinished table, what is the order of increasing acid strength ?
Acid K a p K a Propanoic acid 1.3×10 - 5 Chlorous acid 1.96 Benzoic acid 6.3×10 - 5
(Multiple Choice)
4.9/5  (40)
(40)
What is the pH of aqueous 0.10 M HNO2?
K a(HNO2) = 4.5×10 - 4
(Multiple Choice)
4.7/5  (34)
(34)
What is the Calcium hydroxide concentration, [Ca(OH)2], of an aqueous Ca(OH)2 solution with a pH = 9.60?
(Multiple Choice)
4.9/5  (44)
(44)
A weak monoprotic acid is dissolved in water. A solution that is 0.10 M is found to be 1.2% ionized. Calculate K a for the acid.
(Multiple Choice)
4.9/5  (37)
(37)
A solution of KOH is found to have [OH - ] = 0.010 M. What is the pH of this solution?
(Multiple Choice)
4.9/5  (26)
(26)
Which of the following is considered a weak acid when dissolved in water?
I. HNO2
II. HF
III. H2SO3
(Multiple Choice)
4.8/5  (43)
(43)
Showing 61 - 80 of 179
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)