Exam 15: Solutions of Acids and Bases
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
A dilute solution of HBr is found to have a pH of 3.55. What is [H3O+]?
(Multiple Choice)
4.8/5  (24)
(24)
A 0.250 M aqueous solution of a monoprotic weak acid has [H3O+] of 3.2×10 - 3. What is the analytical concentration of this weak acid?
(Multiple Choice)
4.8/5  (38)
(38)
In the unusual acid-base reaction HClO₄ + H₂ SO₄ 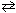 ClO₄- + H ₃SO₄+ , one of the conjugate acid-base pairs is:
ClO₄- + H ₃SO₄+ , one of the conjugate acid-base pairs is:
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following aqueous solutions is(are) considered basic ?
I. [H3O+] = 5.4×10 - 8 M
II. pOH = 9.0
III. [OH - ] = 4.3×10 - 4
(Multiple Choice)
4.9/5  (39)
(39)
Consider the salt ammonium fluoride, NH4F, that is derived from a combination of a weak acid and a weak base as shown below. NH3 (g) + HF (g)→NH4F K b(NH3) = 1.8×10 - 5 and K a(HF) = 6.8×10 - 4 Which statement listed below is true?
(Multiple Choice)
4.8/5  (33)
(33)
Identify the Lewis base in the following reaction. H2O + H - →H2 + OH -
(Multiple Choice)
4.9/5  (30)
(30)
What is the H3O+ concentration in a solution formed by dissolving 63 g of HNO3 in 2.0 liters of water?
[molar mass of HNO3 = 63 g/mol]
(Multiple Choice)
4.8/5  (42)
(42)
Exhibit 15-6 Consider an aqueous 0.25 M solution of NaCN to answer the following question(s).
-Refer to Exhibit 15-6. What is the base ionization constant, K b, for CN - ?
K a(HCN) = 4.9×10 - 10
(Multiple Choice)
4.8/5  (27)
(27)
Which statement below is true about an aqueous solution of potassium fluoride, KF, and why?
(Multiple Choice)
4.7/5  (28)
(28)
A solution is found to have a pH of 10.45. What is [H3O+]?
(Multiple Choice)
4.9/5  (29)
(29)
What is the hydronium ion concentration, [H3O+], of an aqueous 0.083 M hydroxylamine, HONH2, solution?
Hydroxylamine is a basic substance when dissolved in water. K b(HONH2) = 1.1×10 - 8
(Multiple Choice)
4.8/5  (45)
(45)
Calculate the analytical concentration of a solution of acetic acid that has a pH of 3.00 ( K a for acetic acid is 1.8×10 - 5).
(Multiple Choice)
4.7/5  (38)
(38)
Employing a neutralization reaction , what reactants are required to prepare the salt, KNO3 reactants?
→KNO3
(Multiple Choice)
5.0/5  (36)
(36)
When comparing acid strengths, which one of the items listed below is not correctly labeled?
(Multiple Choice)
4.8/5  (36)
(36)
The pH of a certain black coffee is 5.3. What is its hydroxide ion concentration, [OH - ]?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following aqueous solutions is(are) considered basic ?
I. [H3O+] = 3.4×10 - 10 M
II. pH = 8.54
III. [OH - ] = 6.7×10 - 4 M
(Multiple Choice)
4.9/5  (37)
(37)
If we compare the acid/base properties of a 0.5 M solution of A - to a 0.5 M solution of HA, K a = 1.0×10 - 4:
(Multiple Choice)
4.7/5  (36)
(36)
Showing 161 - 179 of 179
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)