Exam 15: Solutions of Acids and Bases
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
Which of the following acids is considered a strong acid when dissolved in water?
I. HNO2 (Nitrous acid)
II. HClO4 (Perchloric acid)
III. HF (Hydrofluoric acid)
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following is a property of a base?
I. Slippery to touch.
II. Tastes bitter.
III. Reacts with active metals to form H2 gas.
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following is considered a strong acid when dissolved in water?
(Multiple Choice)
4.9/5  (43)
(43)
Consider the following Bronsted-Lowery acid-base reaction:
HClO ₂ + N(CH ₃) ₃ 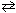 HN(CH ₃) ₃+ + ClO ₂ 1 -Which two substances represent Bronsted-Lowery bases in this reaction?
HN(CH ₃) ₃+ + ClO ₂ 1 -Which two substances represent Bronsted-Lowery bases in this reaction?
(Multiple Choice)
4.8/5  (42)
(42)
Regarding acid strength, which of the following is falsely labeled?
(Multiple Choice)
4.8/5  (35)
(35)
The hydrogen ion concentration of a 0.0010 M solution of hypochlorous acid ( K a = 3.0×10 - 8) is:
(Multiple Choice)
4.7/5  (35)
(35)
Which of the following aqueous solutions is(are) considered basic ?
I. [OH - ] = 6.2×10 - 8
II. [H3O+] = 3.8×10 - 12
III. pOH = 2.5
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following aqueous solutions is(are) considered acidic ?
I. A solution with [OH - ] = 2.5×10 - 8
II. A solution with [H3O+] = 1×10 - 7
III. A solution with a pOH = 8
(Multiple Choice)
4.8/5  (32)
(32)
Calculate the pH of 0.010 M NH4Cl. K b for NH3 is 1.8×10 - 5.
(Multiple Choice)
4.8/5  (35)
(35)
Which acid listed below would be the strongest given the corresponding base ionization constants for their conjugate bases?
(Multiple Choice)
4.9/5  (36)
(36)
Which base listed below would be the strongest given the corresponding acid ionization constants for their conjugate acids?
(Multiple Choice)
5.0/5  (37)
(37)
Consider the acid strengths of the binary hydrides of Group VIA (16) H2O, H2S, H2Se, and H2Te.
(Multiple Choice)
4.7/5  (33)
(33)
Phenylacetic acid is one of the substances that accumulates in the blood of persons with phenylketonuria, an inherited disorder that can cause mental retardation or even death. A 0.085 M solution of this substance is found to have a pH of 2.68. What is the acid ionization constant , K a, for this acid?
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following is considered a strong acid when dissolved in water?
I. HClO2
II. HBr
III. HNO3
(Multiple Choice)
4.9/5  (44)
(44)
Showing 141 - 160 of 179
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)