Exam 16: Reactions Between Acids and Bases
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
In the titration of 25.0 mL of a solution of a weak acid HX with 0.250 M NaOH, the pH = 4.50 after the addition of 5.0 mL of the NaOH solution. The equivalence point occurs when 30.4 mL of the NaOH solution has been added. What is the ionization constant ( K a) of HX?
(Multiple Choice)
4.8/5  (43)
(43)
Carbonic acid is a diprotic acid with K al = 4.3×10 - 7 and K a2 = 5.6×10 - 11. Estimate the pH of a solution of sodium hydrogen carbonate.
(Multiple Choice)
4.8/5  (49)
(49)
Solutions are made by combining equal amounts of the following. Which is a buffer?
(Multiple Choice)
4.9/5  (46)
(46)
Exhibit 16-1 Consider the equilibrium reaction below to answer the following question(s). HNO₂ + H₂ O 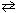 H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4
-Refer to Exhibit 16-1. What is the concentration of nitrite ion, NO₂ - , present at equilibrium if 0.100 moles of HCl are added to 0.200 moles of HNO₂ to form one-liter of solution?
H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4
-Refer to Exhibit 16-1. What is the concentration of nitrite ion, NO₂ - , present at equilibrium if 0.100 moles of HCl are added to 0.200 moles of HNO₂ to form one-liter of solution?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following salts, each having very limited solubility in water, would dissolve to a greater extent upon acidifying the solution?
I. Ag2S
II. Mg3(PO4)2
III. AgBr
(Multiple Choice)
4.9/5  (36)
(36)
Oxalic acid is diprotic with p K al = 1.23 and p K a2 = 4.19. What is p K b for the hydrogen oxalate ion?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following titrations would produce a pH equal to 7.0 at the equivalence point?
I. Titrating NH3 with standard HCl (delivered from the burette)
II. Titrating HCl with standard NaOH (delivered from the burette)
III. Titrating CH3COOH with standard NaOH (delivered from the burette)
(Multiple Choice)
4.8/5  (37)
(37)
A 0.10 M solution of hydrazine (N2H4, K b = 1.7×10 - 6) containing an unknown concentration of hydrazine hydrochloride (N2H5Cl) has a pH of 7.50. What is the concentration of N2H5Cl in the solution?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following general combinations of substances form an acid-base buffer when added to water?
(Multiple Choice)
4.7/5  (47)
(47)
Consider a buffer solution containing a weak acid, HX, and a salt of the weak acid's conjugate base, NaX. Under what conditions is the pH greater than the p K a of the weak acid?
(Multiple Choice)
4.9/5  (32)
(32)
A sample of acetic acid (approximately 0.001 M, K a = 1.8×10 - 5) is titrated with 0.00993 M NaOH. What indicator should be used?
(Multiple Choice)
4.7/5  (41)
(41)
Consider the two buffer solutions prepared below:
Solution A:
[HA] = 0.15 M and [A - ] = 0.25 M Solution B:
[HA] = 1.50 M and [A - ] = 2.50 M Which statement below is true regarding the pH and buffering capacity of these two solutions?
(Multiple Choice)
4.9/5  (37)
(37)
Calculate the pH of a buffer prepared by dissolving 0.10 mol of NH4Cl in 1.00 L of 0.15 M NH3. K b = 1.8×10 - 5 for NH3.
(Multiple Choice)
4.8/5  (45)
(45)
What is the volume of 0.0175 M Ba(OH)2 required to neutralize 10.0 mL of 0.0300 M HCl?
(Multiple Choice)
4.9/5  (33)
(33)
A buffer was prepared such that the pH was 5.20. If the solution contains 0.400 moles of acetic acid (CH3COOH) per liter, what is the concentration of the acetate ion (CH3COO - ) in this solution?
The p K a for acetic acid is 4.74.
(Multiple Choice)
4.9/5  (44)
(44)
Calculate the pH of a solution formed when 1.5 L oF4.5×10 - 3 M HCl is mixed witH₂ .0 L of 3.0×10 - 3 M HNO3.
(Multiple Choice)
4.9/5  (44)
(44)
Which of the following mixtures would not be suitable for preparing a buffer solution?
(Multiple Choice)
4.7/5  (42)
(42)
A sample of 0.100 mole of acetic acid ( K a = 1.8×10 - 5) in 50.0 mL of solution is titrated with standard 0.100 M NaOH. What is the pH in the titration flask after addition of 25.0 mL of the base?
(Multiple Choice)
4.9/5  (38)
(38)
Consider the titration of a weak acid with a strong base . Which statement below is true regarding the equivalence point in this titration?
(Multiple Choice)
4.8/5  (45)
(45)
What volume of 0.200 M H₂ SO₄is required to neutralize 50.0 mL of 0.100 M NaOH?
Assume the chemical equation is:
H₂ SO₄(aq) + 2 NaOH (aq) Na2 SO₄(aq) + 2 H₂ O (  )
)
(Multiple Choice)
4.8/5  (29)
(29)
Showing 21 - 40 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)