Exam 16: Reactions Between Acids and Bases
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
What is the pH of an aqueous solution that contains 0.39 M C6H5COOH and 0.14 M C6H5COONa?
K a(C6H5COOH) = 6.3×10 - 5
(Multiple Choice)
4.7/5  (37)
(37)
Consider the following equilibrium reaction:
NH₃+ H₂ O 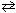 NH ₄+ + OH - K b (NH ₃) = 1.8 10 - 5 Qualitatively, what is the effect of adding NaOH to an aqueous solution of NH₃as shown in the equilibrium reaction above?
(Use Le Chatelier s principle.)
NH ₄+ + OH - K b (NH ₃) = 1.8 10 - 5 Qualitatively, what is the effect of adding NaOH to an aqueous solution of NH₃as shown in the equilibrium reaction above?
(Use Le Chatelier s principle.)
(Multiple Choice)
4.8/5  (27)
(27)
Calculate the pH of a solution that is 0.78 M NH3 and 0.140 M NH4NO3. K b = 1.8×10 - 5 for NH3.
(Multiple Choice)
4.8/5  (38)
(38)
A 25.0 mL volume of 0.100 M aspartic acid (monoprotic) is titrated with 0.100 M NaOH to the equivalence point. If the pH at the equivalence point is 8.28, calculate K a for aspartic acid.
(Multiple Choice)
4.8/5  (41)
(41)
A 25.0 mL volume of a 0.200 M N2H4 solution ( K b = 1.70×10 - 6) is titrated to the equivalence point with 0.100 M HCl. What is the pH of this solution at the equivalence point?
The titration is:
N2H4 + HCl→N2H5+ + Cl -
(Multiple Choice)
4.8/5  (34)
(34)
What is the pH of the following solution?
20)0 mL of 0.50 M acetic acid ( K a = 1.8×10 - 5) is added tO5 .00 mL of 0.50 M NaOH.
(Multiple Choice)
4.8/5  (35)
(35)
If it takes 32.0 mL of 0.100 M HCl to titrate 25.0 mL of a Ba(OH)2 solution to the equivalence point, what is the molarity of the original Ba(OH)2 solution?
(Multiple Choice)
4.7/5  (41)
(41)
Which pair(s) of substances would make a suitable buffer pair combination?
I. NaOH and NaBr
II. HF and NaF
III. CH3NH3+Cl - and CH3NH2
(Multiple Choice)
4.7/5  (33)
(33)
Which one of these combinations would give a buffer that would be most effective in the pH range 3.0 tO4.0?
(Multiple Choice)
4.9/5  (36)
(36)
What is the pH after the addition of 0.050 moles of NaOH to 1.00 L of a 0.500 M NH3 / 0.500 M NH4Cl buffer solution?
Assume that there is no volume change on addition of the NaOH. K b for NH3 = 1.8×10 - 5.
(Multiple Choice)
4.8/5  (36)
(36)
Oxalic acid is diprotic with p K al = 1.23 and p K a2 = 4.19. Estimate the pH of a solution of sodium hydrogen oxalate.
(Multiple Choice)
4.8/5  (29)
(29)
The following compounds have very limited solubility in water. Which of the following would dissolve in water under acidic conditions?
(Multiple Choice)
5.0/5  (37)
(37)
Which of the following titration curves listed below best represents a curve for the complete titration of citric acid, H3C6H5O7, a triprotic acid with a strong base such as NaOH?
(Multiple Choice)
4.8/5  (30)
(30)
Calculate the pH in a solution which is made when 20.0 mL of 0.10 M NH3 ( K b = 1.8×10 - 5) is added to 30.0 mL of 0.10 M HCl.
(Multiple Choice)
4.8/5  (41)
(41)
A solution of HCOOH ( K a = 1.8×10 - 4) and HCOO - was submitted for chemical analysis. The results were:
[HCOOH] = 0.050 M, [HCOO - ] = 0.15 M. Calculate the pH.
(Multiple Choice)
4.8/5  (40)
(40)
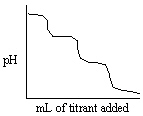
Which experiment listed below would provide a titration curve that resembles the titration shown below?
(Multiple Choice)
5.0/5  (33)
(33)
Consider a buffer solution containing a weak acid, HX, and a salt of the weak acid's conjugate base, NaX. Under what conditions does the pH just equal the p K a of the weak acid?
(Multiple Choice)
4.7/5  (29)
(29)
What is the pH of an aqueous solution that contains 0.085 M HNO2 and 0.10 M potassium nitrite (KNO2)?
K a(HNO2) = 4.5×10 - 4
(Multiple Choice)
5.0/5  (31)
(31)
Exhibit 16-2 Consider titrating CH3COOH with standard NaOH (delivered from the burette) to answer the following question(s).
-Refer to Exhibit 16-2. How would the pH be determined for the aqueous solution in the titration above at the equivalence point ?
(Multiple Choice)
4.8/5  (34)
(34)
What is the pH of an aqueous solution that contains 0.183 M sodium formate (NaCHO2) and 0.300 M in formic acid?
K a(HCHO2) = 1.8×10 - 4
(Multiple Choice)
4.9/5  (38)
(38)
Showing 41 - 60 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)