Exam 16: Reactions Between Acids and Bases
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
During a titration, 60.0 mL of 0.010 M NaOH is added to 35.0 mL of 0.020 M HCl solution. What is the pH of the resulting solution?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following is a polyprotic acid relative to water?
(Multiple Choice)
4.9/5  (30)
(30)
What is the pH of a solution of 25.0 mL of 0.250 M HNO3 after the addition oF40.0 mL of 0.300 M KOH?
(Multiple Choice)
4.7/5  (34)
(34)
Exhibit 16-1 Consider the equilibrium reaction below to answer the following question(s). HNO₂ + H₂ O  H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4
-Refer to Exhibit 16-1. What is the effect of adding NaNO₂ to an aqueous solution of HNO₂ as shown in the equilibrium reaction above?
H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4
-Refer to Exhibit 16-1. What is the effect of adding NaNO₂ to an aqueous solution of HNO₂ as shown in the equilibrium reaction above?
(Multiple Choice)
4.7/5  (38)
(38)
What is the molarity of Ba(OH)2 solution if 65.0 mL of 0.0500 M HCl is required to titrate 35.00 mL of the Ba(OH)2 solution?
(Multiple Choice)
4.9/5  (43)
(43)
In the titration of 0.100 M HCl with the titrant 0.100 M NaOH, what species are present at the equivalence point?
(Multiple Choice)
4.8/5  (29)
(29)
Consider the ionization of hydrofluoric acid as follows:
HF + H₂ O  H₃O + + F 1 - What is the effect of adding the common ion, F - (source:
NaF), to this equilibrium system?
H₃O + + F 1 - What is the effect of adding the common ion, F - (source:
NaF), to this equilibrium system?
(Multiple Choice)
4.9/5  (44)
(44)
Calculate the amount of ammonium chloride (NH4Cl) that must be added to 1.0 L of 0.10 M NH3 ( K b = 1.8×10 - 5) to prepare a buffer of pH 9.00.
(Multiple Choice)
4.7/5  (31)
(31)
A sample of 100 mL of 0.10 M acid HA ( K a = 1.0×10 - 5) is titrated with standard 0.100 M KOH. How many mL of KOH will have been added when the pH in the titration flask is 5.00?
(Multiple Choice)
4.8/5  (43)
(43)
What is the pH of an aqueous solution that is 0.55 M HNO2 and 0.75 M KNO2?
K a(HNO2) = 7.1×10 - 4
(Multiple Choice)
4.9/5  (36)
(36)
A sample of 50.0 mL of 0.10 M NH3 ( K b = 1.8×10 - 5) is titrated with 0.10 M HCl. Calculate the pH at the equivalence point.
(Multiple Choice)
4.9/5  (32)
(32)
Exhibit 16-3 Consider the Titration curve below for the titration of a weak acid ,CH ₃COOH , with a strong base , NaOH, to answer the following question(s). CH ₃COOH + NaOH CH ₃COONa + H₂ O 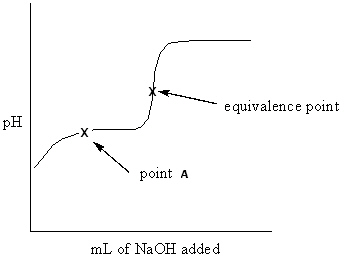 Refer to Exhibit 16-3. What approach listed below would be used to solve for the pH at the equivalence point on this titration curve?
Refer to Exhibit 16-3. What approach listed below would be used to solve for the pH at the equivalence point on this titration curve?
(Multiple Choice)
4.9/5  (36)
(36)
A buffer was prepared by mixing 0.60 moles of HF and 0.40 moles of NaF in enough water to make 1.00 L of solution. What is the pH of this solution?
K a = 3.5×10 - 4 for HF.
(Multiple Choice)
4.9/5  (37)
(37)
A 0.40 gram sample of NaOH (molar mass = 40.0 g/mol) was completely neutralized witH₆2.5 mL of H₂ SO₄solution. Assume the chemical equation is:
H₂ SO₄(aq) + 2 NaOH (aq) Na2 SO₄(aq) + 2 H₂ O (  ) The molarity of the H₂ SO₄solution was:
) The molarity of the H₂ SO₄solution was:
(Multiple Choice)
5.0/5  (33)
(33)
The titration of a weak acid, HA ( K a = 1.0×10 - 4), with NaOH requires 40.0 mL to reach the equivalence point. In order to calculate the pH of the system after 20 mL of NaOH is added, the best strategy is to recognize that the system is
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following would not make a suitable buffer pair?
(Multiple Choice)
4.8/5  (34)
(34)
Calculate the pH of a solution made by mixing 20.00 mL of 0.300 M HCl and 50.00 mL of 0.100 M NaOH and diluting to a final volume of 1.00 L.
(Multiple Choice)
4.7/5  (29)
(29)
A 25.0 mL portion of 0.0500 M HCl was added to 10.0 mL of 0.0250 M NaOH. Calculate the pH of the resulting solution.
(Multiple Choice)
4.8/5  (35)
(35)
A buffer solution maintains a constant pH level to a certain extent when a strong base such as NaOH is added. Which of the following buffer solutions has the greatest buffering capacity to consume added NaOH?
(Multiple Choice)
4.9/5  (33)
(33)
Consider the buffer pair, NH4Cl/NH3. What can be stated about the relative proportions of this buffer pair if the pH of an aqueous solution of this pair is adjusted to 12.00 and the p K a for NH4+ equals 9.25?
(Multiple Choice)
4.8/5  (43)
(43)
Showing 61 - 80 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)