Exam 16: Reactions Between Acids and Bases
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
What is the pH after the addition of 0.20 mole of HCl to 1.00 liter of 1.00 M CH3COONa and 1.00 M CH3COOH ( K a = 1.8×10 - 5) buffer solution?
(Assume no volume change.)
(Multiple Choice)
4.9/5  (38)
(38)
The pH at the equivalence point in the titration of 0.10 M NH3 with 0.10 M HCl is:
(Multiple Choice)
4.8/5  (33)
(33)
How many grams of sodium acetate, CH3COONa, is required to mix witH₂ .00 Liters of 0.450 M acetic acid, CH3COOH, in order to prepare a buffer solution with a pH that equals 5.00?
P K a(CH3COOH) = 4.74 and MM(CH3COONa) = 82.03 g/mol
(Multiple Choice)
4.8/5  (40)
(40)
A sample of ammonia (approximately 0.10 M, K b = 1.8×10 - 5) is titrated with 0.0993 M HCl. What indicator should be used?
(Multiple Choice)
4.8/5  (33)
(33)
In the titration of 25.0 mL of 0.100 M acetic acid ( K a = 1.8×10 - 5) with 0.100 M NaOH, the pH after adding 12.5 mL of titrant is:
(Multiple Choice)
4.7/5  (30)
(30)
What is the pH of a buffer solution that consists of 0.83 M HBrO and 0.53 M KBrO?
K a(HBrO) = 2.3×10 - 9
(Multiple Choice)
4.9/5  (34)
(34)
What is the concentration of ammonium ion, [NH4+], present at equilibrium if 0.100 moles of NaOH are added to 0.200 moles of NH3 to form one-liter of solution?
(Multiple Choice)
4.7/5  (28)
(28)
The sharpest inflection point is observed in the titration of
(Multiple Choice)
4.8/5  (25)
(25)
In the titration of 0.100 M HCl with the titrant 0.100 M NaOH, what species are present after the equivalence point?
(Multiple Choice)
4.9/5  (39)
(39)
The titration of a weak acid, HA ( K a = 1.0×10 - 4), with NaOH requires 40.0 mL to reach the equivalence point. The pH after 20 mL is
(Multiple Choice)
4.8/5  (40)
(40)
What is the pH of solution formed by adding 25.0 mL of 0.100 M HCl to 25.0 mL of 0.100 M pyridine (C5H5N, K b = 1.8×10 - 9)?
(Multiple Choice)
4.9/5  (39)
(39)
Consider the ionization of acetic acid as follows:CH ₃COOH + H₂ O  H₃O + + CH₃COO 1 - What is the effect of adding the common ion, acetate, CH₃COO - (source:
CH₃COONa), to this equilibrium system?
H₃O + + CH₃COO 1 - What is the effect of adding the common ion, acetate, CH₃COO - (source:
CH₃COONa), to this equilibrium system?
(Multiple Choice)
4.8/5  (34)
(34)
Exhibit 16-3 Consider the Titration curve below for the titration of a weak acid ,CH ₃COOH , with a strong base , NaOH, to answer the following question(s). CH ₃COOH + NaOH CH ₃COONa + H₂ O 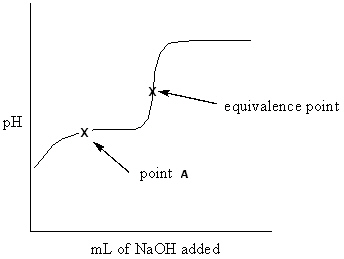 Refer to Exhibit 16-3. What approach listed below would be used to determine the pH at point A of the solution?
Refer to Exhibit 16-3. What approach listed below would be used to determine the pH at point A of the solution?
(Multiple Choice)
4.9/5  (31)
(31)
A solution is made by dissolving 0.100 mole NH3 ( K b = 1.8×10 - 5) and 0.200 mole of NH4Cl in water and diluting to 1.00 L. What is the pH of the resulting solution?
(Multiple Choice)
4.7/5  (43)
(43)
Calculate the pH at the equivalence point for the titration of 20.0 mL of 1.00 M HF with 0.500 M NaOH. K a = 3.5×10 - 4 for HF.
(Multiple Choice)
4.9/5  (42)
(42)
Carbonic acid is a diprotic acid with K al = 4.3×10 - 7 and K a2 = 5.6×10 - 11. What is K b for the carbonate ion, CO32 - ?
(Multiple Choice)
5.0/5  (39)
(39)
A sample of hydrochloric acid (approximately 0.10 M) is titrated with 0.0993 M NaOH. What indicator changes color closest to the equivalence point?
(Multiple Choice)
4.8/5  (32)
(32)
The titration of a weak base ( K b = 1.0×10 - 4) with HCl requires 40.0 mL to reach the equivalence point. In order to calculate the pH of the system after 20 mL of HCl is added, the best strategy is to recognize that the system is
(Multiple Choice)
4.7/5  (32)
(32)
Showing 81 - 98 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)