Exam 2: Atoms, Elements, and Compounds
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds77 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Energy and Physical Properties74 Questions
Exam 5: Solution Concentration77 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases85 Questions
Exam 8: Nuclear Chemistry67 Questions
Exam 9: Hydrocarbons: an Introduction to Organic Molecules74 Questions
Exam 10: Hydration, Dehydration, and Alcohols61 Questions
Exam 11: Carbonyl Compounds and Redox Reactions71 Questions
Exam 12: Organic Acids and Bases64 Questions
Exam 13: Condensation and Hydrolysis Reactions72 Questions
Exam 14: Proteins67 Questions
Exam 15: Carbohydrates75 Questions
Exam 16: Lipids and Membranes79 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity71 Questions
Select questions type
Compound consists of five atoms of carbon, ten atoms of hydrogen, and five atoms of oxygen. The formula for this compound is
(Multiple Choice)
4.9/5  (32)
(32)
Based on the text periodic table and using the correct number of significant figures, enter the appropriate number in the blank. There are _______mol is 72.36 g of citric acid, C6H8O7.
(Short Answer)
4.7/5  (36)
(36)
Fill in each blank with the appropriate term from the list given below.
protons
neutrons
electrons
valence electrons
-14N and 15O have the same number of _____________________.
(Short Answer)
4.7/5  (31)
(31)
Classify the following statement as representing an intensive or extensive property by placing intensive or extensive in the blank. "Vinegar tastes sour.": _________________
(Short Answer)
4.8/5  (44)
(44)
Enter an integer number (1, 2, 3, ...) in the blank. Sucrose (table sugar) has the formula C12H22O11. How many oxygen atoms would be in 3 formula units of sucrose?
____________________oxygen atoms.
(Short Answer)
4.8/5  (38)
(38)
Fill in each blank with the appropriate term from the list given below.
protons
neutrons
electrons
valence electrons
-N, P and As have the same number of_____________________.
(Short Answer)
4.8/5  (41)
(41)
A mole of an element contains the same number of atoms as a mole of any other element.
(True/False)
4.9/5  (38)
(38)
Convert the following number of moles into the corresponding mass. 5.22 mol Br
(Multiple Choice)
4.8/5  (40)
(40)
The pie chart shown below represents the elemental composition of the human body including water. 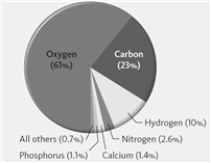
(True/False)
4.8/5  (34)
(34)
Use the following terms to complete the two statements given. A term may be used more than once.
heterogeneous mixture
homogeneous mixture
compound
In a restaurant fresh ground pepper is added to olive oil as a dipping sauce for bread. This dip represents a_____________________. Coffee with caramel flavoring is served for dessert. The coffee is an example of a _______________________.
(Short Answer)
4.7/5  (28)
(28)
One of the orbitals in a shell of an atom could be pictured as shown below. 
(True/False)
4.9/5  (36)
(36)
Which of the following is the conversion factor that could be used to convert a mass in grams of sodium to the corresponding number of moles?
(Multiple Choice)
5.0/5  (35)
(35)
The statement below describes an intensive property.  "The cost of this container of milk is $2.99/gal."
"The cost of this container of milk is $2.99/gal."
(True/False)
4.8/5  (25)
(25)
Convert the following mass moles. 22.98 g glycine, an amino acid, C2H5NO2.
(Short Answer)
4.9/5  (41)
(41)
Which of the following is true of the atomic weight of an element?
(Multiple Choice)
4.9/5  (40)
(40)
Showing 41 - 60 of 77
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)