Exam 2: Atoms, Elements, and Compounds
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds77 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Energy and Physical Properties74 Questions
Exam 5: Solution Concentration77 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases85 Questions
Exam 8: Nuclear Chemistry67 Questions
Exam 9: Hydrocarbons: an Introduction to Organic Molecules74 Questions
Exam 10: Hydration, Dehydration, and Alcohols61 Questions
Exam 11: Carbonyl Compounds and Redox Reactions71 Questions
Exam 12: Organic Acids and Bases64 Questions
Exam 13: Condensation and Hydrolysis Reactions72 Questions
Exam 14: Proteins67 Questions
Exam 15: Carbohydrates75 Questions
Exam 16: Lipids and Membranes79 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity71 Questions
Select questions type
When a particular solid sample is examined under a microscope, it is observed that there are regions that are black and regions which are yellow. What type of matter is this sample?
(Multiple Choice)
4.7/5  (38)
(38)
Write the chemical symbol for the elements that is in period 2 and group 8A of the periodic table.
(Short Answer)
4.9/5  (35)
(35)
The electron arrangement for Na would be:
shell 1: 2 electrons shell 2: 8 electrons shell 3: 1 electron
(True/False)
4.8/5  (34)
(34)
Based on the text periodic table and using the correct number of significant figures, enter the appropriate number in the blank. The mass of 7.00 mol of sodium chloride, NaCl, is _____________g.
(Short Answer)
4.9/5  (43)
(43)
The atomic weight of phosphorus (P) is 30.91 amu or about 31 amu. This indicates that each P atom consists of 15 protons and 16 neutrons.
(True/False)
4.8/5  (36)
(36)
Sodium is a highly reactive metal and chlorine is a toxic gas, but when they come together the resulting material, sodium chloride (a white solid), is essential for life. Which of the following is true when sodium and chlorine are brought into contact with one another?
(Multiple Choice)
4.8/5  (35)
(35)
Enter the chemical symbol of the element in the blank. An element in period 3 has the Lewis structure: 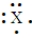 X should be replaced with what chemical symbol?
Chemical symbol:_____________________.
X should be replaced with what chemical symbol?
Chemical symbol:_____________________.
(Short Answer)
4.7/5  (36)
(36)
A person drinks 1900 g of water, H2O, per day. How many moles of water did they consume?
(Multiple Choice)
4.9/5  (37)
(37)
Consider the atomic view of a substance shown below.  This substance would be classified as a(n)
This substance would be classified as a(n)
(Multiple Choice)
4.7/5  (40)
(40)
Enter the chemical symbol of the element in the blank. An element has the following electron arrangement. shell 1: 2 electrons shell 2: 8 electrons shell 3: 18 electrons shell 4: 18 electrons shell 5: 4 electrons
The reactivity of this element would most closely resemble that of which element?
Chemical symbol:_____________________
(Short Answer)
4.8/5  (37)
(37)
Indicate the number of dots that should be placed around the Lewis symbol for the following element. Use an integer: 1, 2, 3, etc. as appropriate. Br
(Short Answer)
4.8/5  (23)
(23)
Neutral isotopes of the same element have the same number of electrons.
(True/False)
4.9/5  (44)
(44)
What is the meaning of the subscripts in the formula for ethyl alcohol, C2H5OH?
(Multiple Choice)
4.9/5  (32)
(32)
Naturally occurring lithium (Li) consists of only two isotopes, Li-6 (6.02 amu) and Li-7 (7.02 amu), where the exact isotopic masses are given in parentheses. Use the periodic table and determine which isotope is present in the larger percentage in the natural element.
(Multiple Choice)
5.0/5  (30)
(30)
Electron shells define a region in space around the nucleus occupied by certain electrons.
(True/False)
4.9/5  (41)
(41)
An elements with an electron arrangement of:
shell 1: 2 electrons shell 2: 8 electrons shell 3: 18 electrons shell 4: 18 electrons shell 5: 7 electrons
would have 1 valence electron.
(True/False)
4.8/5  (32)
(32)
Use the periodic table to determine about how many helium atoms (He) on the average would be needed to get close to the same mass as an oxygen atom (O).
(Multiple Choice)
4.9/5  (39)
(39)
Based on the text periodic table and using the correct number of significant figures, enter the appropriate number in the blank. There are _______mol is 54.05 g of boron.
(Short Answer)
4.8/5  (43)
(43)
Sodium chlorate, an ingredient in many common herbicides, has sodium, chlorine and oxygen atoms in the ratio 1:1:3, respectively. What is the formula unit for sodium chlorate?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 21 - 40 of 77
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)