Exam 2: Atoms, Elements, and Compounds
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds77 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Energy and Physical Properties74 Questions
Exam 5: Solution Concentration77 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases85 Questions
Exam 8: Nuclear Chemistry67 Questions
Exam 9: Hydrocarbons: an Introduction to Organic Molecules74 Questions
Exam 10: Hydration, Dehydration, and Alcohols61 Questions
Exam 11: Carbonyl Compounds and Redox Reactions71 Questions
Exam 12: Organic Acids and Bases64 Questions
Exam 13: Condensation and Hydrolysis Reactions72 Questions
Exam 14: Proteins67 Questions
Exam 15: Carbohydrates75 Questions
Exam 16: Lipids and Membranes79 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity71 Questions
Select questions type
Group 4A and Group 14 are two different designations for the same column of the periodic table.
(True/False)
4.8/5  (32)
(32)
Enter the chemical symbol of the element in the blank. Calcium forms the compound CaF2 . What other alkaline earth element with a smaller atomic mass would form a compound with a similar formula?
Chemical symbol:_____________________.
(Short Answer)
4.9/5  (44)
(44)
The elements sodium, potassium, and oxygen are considered to be "elements of life" and are in the category of electrolytes.
(True/False)
4.8/5  (38)
(38)
Use one the following terms to complete the given statement.
salt
sugar
vegetable oil
When ____________________ is added to water a heterogeneous mixture forms.
(Short Answer)
4.8/5  (46)
(46)
An element is a shiny gray solid that can be pressed into a thin sheet. This element is probably a metalloid.
(True/False)
4.8/5  (48)
(48)
Based on the text periodic table and using the correct number of significant figures, enter the appropriate number in the blank. The mass of 5.00 mol of oxygen atoms is _____________g.
(Short Answer)
4.8/5  (38)
(38)
Which of the following is the correct unit for the formula weight of a large number of atoms?
(Multiple Choice)
4.8/5  (34)
(34)
Fill in each blank with the appropriate term from the list given below.
protons
neutrons
electrons
valence electrons
-14N and 15N
have the same number of _____________________.
(Short Answer)
4.9/5  (29)
(29)
Elements in the same period of the periodic table generally show similar chemical behavior.
(True/False)
5.0/5  (30)
(30)
Which of the following correctly describes a proton on a subatomic scale?
(Multiple Choice)
4.8/5  (42)
(42)
An element has the following electron arrangement. shell 1: 2 electrons shell 2: 8 electrons shell 3: 18 electrons shell 4: 18 electrons shell 5: 7 electrons
The Lewis structure for this element would be: 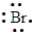
(True/False)
4.9/5  (31)
(31)
Enter an integer number (1, 2, 3, ...) in the blank. Sucrose (table sugar) has the formula C12H22O11. A sample of sucrose contains 2400 carbon atoms. How many formula units does this represent?
____________________formula units.
(Short Answer)
4.9/5  (31)
(31)
A sealed cylinder is filled with a large collection of atoms that have 14 neutrons and 13 protons. Which of the following would behave in a manner similar to this collection of atoms?
(Multiple Choice)
4.7/5  (35)
(35)
An isotope of gallium consisting of 31 protons and 37 neutrons can be represented using the symbol shown below. gallium-37
(True/False)
4.9/5  (39)
(39)
Enter an integer number (1, 2, 3, ...) in the blank. How many electrons are in shell 2 of a P atom?
____________________electrons
(Short Answer)
4.8/5  (40)
(40)
Showing 61 - 77 of 77
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)