Exam 18: Solubility and Simultaneous Equilibria
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
NaOH is added to sea water containing 0.050 moles per liter of Mg2+ ion until the pH reaches 12.0. A certain quantity of Mg(OH)2 precipitates. How many parts per billion of magnesium are left behind in the solution? The Ksp of Mg(OH)2 is 7.1 × 10-12.Hint: Find [Mg2+], then convert to ppb.
(Multiple Choice)
4.8/5  (33)
(33)
Calculate the minimum concentration of Ag+ ion that must be added to (or built up in)a 0.140 M Na2CrO4 solution, in order to initiate a precipitation of silver chromate. The Ksp of Ag2CrO4 is 1.2 × 10-12.Hint: Use an ICE table to help organize your given information and remember to account for the presence of a common ion.
(Multiple Choice)
4.8/5  (36)
(36)
Addition of a common ion to a solution of a slightly soluble salt will ________ the solubility of the slightly soluble salt.
(Short Answer)
5.0/5  (32)
(32)
200 mL of an aqueous solution contains 0.030 M concentrations of both Pb2+ and Ag+. If 100 mL of 6.0 × 10-2 M NaCl is added to this solution will a precipitate form? If so, what will the precipitate be? The Ksp values for PbCl2 and AgCl are 1.7 × 10-5 and 1.8 × 10-10.Hint: Remember that the solutions are being diluted and use the diluted concentrations.
(Short Answer)
4.8/5  (36)
(36)
What is the solubility, in moles per liter, of AgCl (Ksp = 1.8 × 10-10), in 0.0300 M CaCl2 solution?Hint: Be sure to account for the presence of a common ion and carefully organize. You should also be able to simplify the math because the solubility is very low.
(Multiple Choice)
4.9/5  (25)
(25)
The oxide ion can exist in aqueous solutions provided the pH is higher than 11.00.
(True/False)
4.9/5  (37)
(37)
The solubility of silver phosphate, Ag3PO4, can be expressed in terms of the resulting ion concentrations. Which relationship is correct?
(Multiple Choice)
4.8/5  (41)
(41)
The solubility product of barium fluoride (BaF2)is 1.7 × 10-6. Calculate the concentration of fluoride ions in a saturated solution of barium fluoride.
(Multiple Choice)
4.8/5  (34)
(34)
What is the solubility, in moles per liter, of PbSO4 (Ksp = 6.3 × 10-7)in 0.0230 molar MgSO4 solution?Hint: Be sure to account for the presence of a common ion. You should also be able to simplify the math, because the solubility is very low.
(Multiple Choice)
4.9/5  (33)
(33)
Given the following information 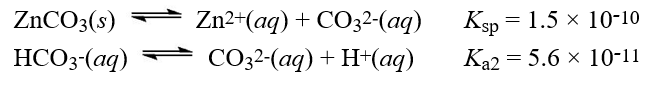 What is the equilibrium constant for the reaction below?
What is the equilibrium constant for the reaction below? 
(Multiple Choice)
4.9/5  (38)
(38)
A solution contains Ba2+ (1.0 × 10-3 M), Ca2+ (1.0 × 10-3 M)and K+ (1.0 × 10-3 M). Drops of a 5.0 × 10-1 M solution of NaF were added through a microburet until the [F-] in the solution mix reached 1.5 × 10-3 M. Should a precipitate form? If so, what is the precipitate? The Ksp values are BaF2: 1.0 × 10-6, CaF2: 5.3 × 10-9. (Neglect the slight change in volume resulting from the added NaF solution.)
(Multiple Choice)
4.9/5  (48)
(48)
The solubility of silver carbonate, Ag2CO3, in pure water is 1.27 × 10-4 moles per liter. Calculate the value of Ksp for silver carbonate from this data.
(Multiple Choice)
4.8/5  (41)
(41)
The Ksp value for barium chromate is 2.1 × 10-10. A 4.16 g sample of BaCl2 and a 5.83 g sample of potassium chromate were added to a 1.000 liter volumetric flask, and distilled water was added to the mark. After placing the stopper and shaking the flask to dissolve as much chemicals as would dissolve, what would be the barium ion concentration remaining in solution afterwards? Be mindful that a precipitate may be formed.Hint: Solving this problem requires comparing Q vs Ksp.
(Multiple Choice)
4.8/5  (42)
(42)
The solubility of lead iodide is 578 mg L-1 at 25 °C. What is the Ksp for PbI2?
(Multiple Choice)
4.8/5  (35)
(35)
A precipitate will form when a solution containing a cation and a solution containing an anion are mixed if the ________ exceeds the value of the ________.
(Short Answer)
4.7/5  (34)
(34)
Which one of the following salts has the highest solubility in water, expressed in moles per liter?
(Multiple Choice)
4.9/5  (41)
(41)
Calculate the concentration of chloride ions in a saturated lead(II)chloride solution. The Ksp = 2.4 × 10-4 for lead(II)chloride.
(Short Answer)
4.9/5  (41)
(41)
The solubility of zinc(II)phosphate, Zn3(PO4)2, in pure water is 1.5 × 10-7 moles per liter. Calculate the value of Ksp for zinc(II)phosphate from this data.
(Multiple Choice)
4.9/5  (41)
(41)
The formation constant for the tris(ethylenediammine)nickel(II)complex ion, [Ni(en)3]2+, is 4.1 × 1017. What is the concentration of the free nickel(II)ion in a solution in which the equilibrium concentration of free ethylenediammine is 0.400 M, and that of the nickel complex above is 0.0100 M?
(Short Answer)
4.7/5  (42)
(42)
The solubility of calcium oxalate, CaC2O4, in pure water is 4.8 × 10-5 moles per liter. Calculate the value of Ksp for calcium oxalate from this data.
(Multiple Choice)
4.9/5  (33)
(33)
Showing 21 - 40 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)