Exam 18: Solubility and Simultaneous Equilibria
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
Mercury ions (Hg2+)are very difficult to dissolve in a solution of water, or even a dilute solution of NaOH. But if ammonia is added to the water, mercury ions are more readily soluble. Which of the following best explains why this happens?
(Multiple Choice)
4.8/5  (45)
(45)
Methylamine, CH3NH2, is a weak molecular base with a value of 4.4 × 10-4 for Kb. An aqueous solution contains 0.200 M CH3NH2 and 0.400 M CH3NH3Cl per liter as the only solutes. If the Ksp of Mg(OH)2 is 7.1 × 10-12, what is the maximum [Mg2+] that can coexist with these solutes in the solution?Hint: Use the Henderson-Hasselbalch formula to find the pOH of the solution, and then use that to find [OH-].
(Multiple Choice)
4.9/5  (36)
(36)
The solubility of magnesium carbonate, MgCO3, in pure water is 2.6 × 10-4 moles per liter. Calculate the value of Ksp for magnesium carbonate from this data.
(Multiple Choice)
4.9/5  (32)
(32)
Will a precipitate form when 20.0 mL of 1.8 × 10-3 M Pb(NO3)2 is added to 30.0 mL of5.0 × 10-4 M Na2SO4? The Ksp of (PbSO4)is 6.3 × 10-7.Hint: Find the concentration of each ion in the insoluble compound, and use those to find Q.
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following is the expression for the solubility product of calcium fluoride, CaF2?
(Multiple Choice)
4.8/5  (43)
(43)
The solubility of barium carbonate is 14.8 mg L-1 at 30 °C. Calculate the Ksp value for BaCO3.
(Multiple Choice)
4.8/5  (31)
(31)
200 mL of an aqueous solution contains 0.030 M concentrations of both Pb2+ and Ag+. If 100 mL of 0.20 M NaCl is added to this solution will a precipitate form? If so, what will the precipitate be? The Ksp values for PbCl2 and AgCl are 1.7 × 10-5 and 1.8 × 10-10.Hint: Remember that the solutions are being diluted and use the diluted concentrations.
(Short Answer)
4.8/5  (32)
(32)
What is the maximum concentration of Mg2+ ion that can exist in a 0.10 M NaF(aq)solution, without precipitating any magnesium fluoride? The Ksp of MgF2 is 6.6 × 10-9.
(Multiple Choice)
4.9/5  (35)
(35)
Given the following information: 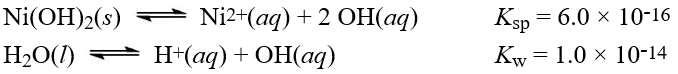 What is the equilibrium constant for the reaction below?
What is the equilibrium constant for the reaction below? 
(Multiple Choice)
4.9/5  (45)
(45)
The sulfide ion is too basic to exist as such in aqueous solutions.
(True/False)
4.7/5  (34)
(34)
Methylamine, CH3NH2, is a weak molecular base with a value of 4.4 × 10-4 for Kb. An aqueous solution contains 0.200 M CH3NH2 and 0.300 M CH3NH3Cl per liter as the only solutes. If the Ksp of Fe(OH)2 is 7.9 × 10-16, what is the maximum [Fe2+] that can coexist with these solutes in the solution?Hint: Use the Henderson-Hasselbalch formula to find the pOH of the solution, and then use that to find [OH-].
(Multiple Choice)
4.9/5  (43)
(43)
The formation constant for the diammine silver(I)ion is 1.6 × 107, while the solubility product constant for silver chloride is 1.8 × 1010. What is the equilibrium constant for the reaction,AgCl(s)+ 2 NH3(aq)  Ag(NH3)2+(aq)+ Cl(aq)?
Ag(NH3)2+(aq)+ Cl(aq)?
(Short Answer)
4.9/5  (39)
(39)
Metal cations in the chloride solubility group can be separated from other cations because they form ________ chloride compounds in a solution of HCl.
(Short Answer)
4.9/5  (30)
(30)
The solubility product for Ag3PO4 is: Ksp = 2.8 × 10-18. What is the solubility of Ag3PO4 in water, in grams per liter?
(Multiple Choice)
4.7/5  (36)
(36)
Even though silver chloride is only very slightly soluble in water, addition of a reagent which forms complex ions with silver ions increases this solubility.
(True/False)
4.8/5  (35)
(35)
The Ksp value for barium chromate is 2.1 × 10-10. A 4.16 g sample of BaCl2 and a 5.83 g sample of potassium chromate were added to a 1.000 liter volumetric flask, and distilled water was added to the mark. After placing the stopper and shaking the flask to dissolve as much chemicals as would dissolve, how many grams of precipitate, if any, would be formed?Hint: Solving this problem requires comparing Q vs Ksp.
(Multiple Choice)
4.8/5  (31)
(31)
The solubility of strontium fluoride, SrF2, can be expressed in terms of the resulting ion concentrations. Which relationship is correct?
(Multiple Choice)
4.8/5  (37)
(37)
What is the solubility, in moles per liter, of AgCl (Ksp = 1.8 × 10-10), in 0.0100 molar aqueous potassium chloride solution?Hint: Be sure to account for the presence of a common ion. You should also be able to simplify the math because the solubility is very low.
(Multiple Choice)
5.0/5  (45)
(45)
Which one of the compounds below has the lowest solubility in water, expressed in moles per liter?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 41 - 60 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)